When it comes to enhancing the properties of copper, two popular choices stand out: nickel plating and tin plating. Both methods offer distinct advantages, but how do they truly stack up against each other? Whether you’re looking for superior corrosion resistance, improved electrical conductivity, or optimal high-temperature performance, understanding the nuances of these plating processes is crucial. In this article, we’ll dive deep into the key differences between nickel and tin plating on copper, comparing their mechanical properties, effectiveness in various applications, and overall cost implications. So, which plating method will emerge as the best choice for your needs? Let’s explore the intricate details and find out.
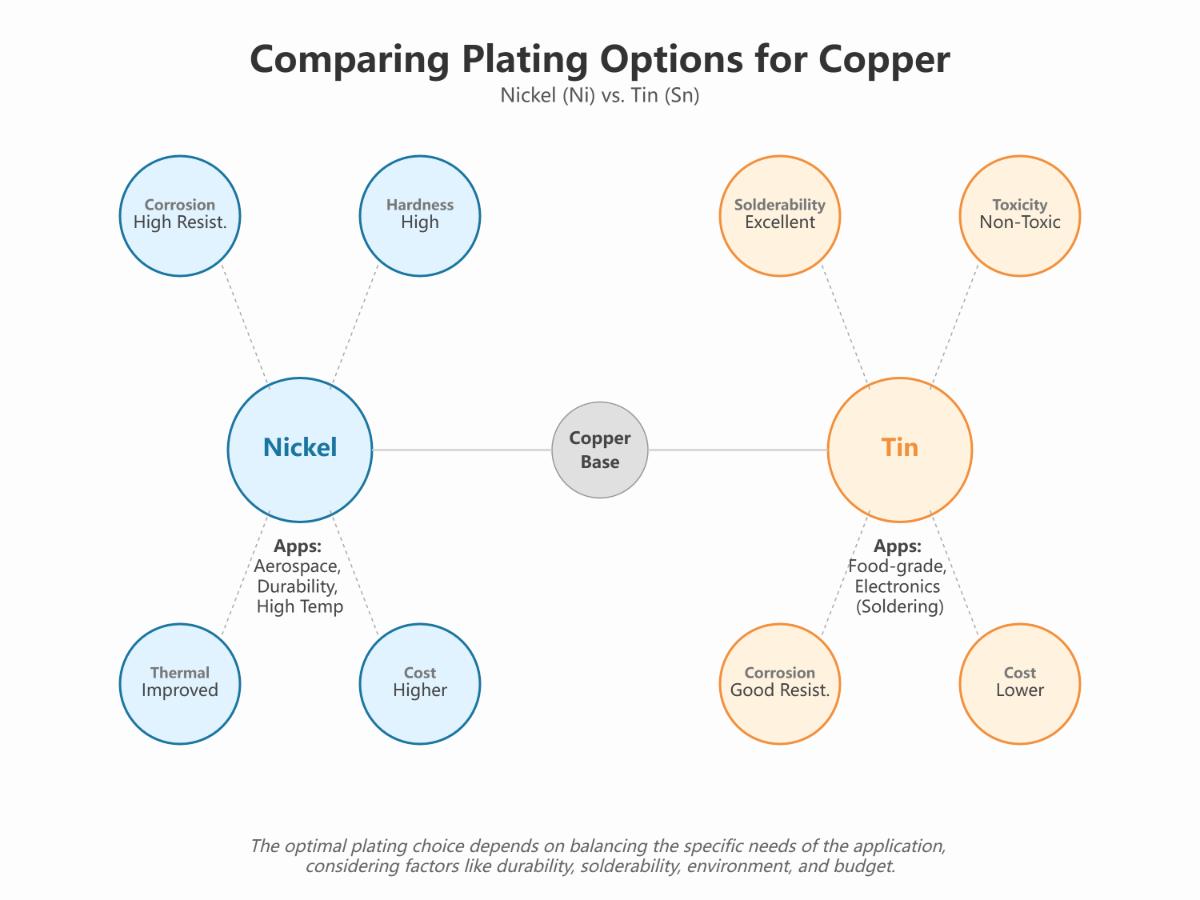
Overview of Nickel Plating and Tin Plating Processes on Copper
Nickel Plating on Copper
Nickel plating is a common technique used to enhance the properties of copper, offering significant benefits for various applications. The process can be divided into two main types: electroplating and electroless plating.
Electroplating Process
In electroplating, an electric current is used to deposit nickel ions onto the copper surface. This technique requires an electrolyte solution containing nickel salts and an anode made of nickel. The copper substrate acts as the cathode, where nickel ions are reduced and form a uniform nickel layer.
Electroless Plating Process
Electroless plating, unlike electroplating, does not require an electric current. Instead, it uses chemical agents in an aqueous solution to deposit nickel onto the copper surface, achieving a more uniform coating, even on complex geometries.
Benefits of Nickel Plating
Nickel plating offers several benefits:
- Corrosion Resistance: Nickel plating significantly enhances the corrosion resistance of copper, making it suitable for environments with high moisture levels.
- Operating Temperature: Nickel plating increases the operating temperature of copper, which is beneficial for applications involving high thermal demands, such as electric motor parts and piping.
- Electrical Conductivity: Though nickel is less conductive than copper, the plating can maintain or slightly improve the electrical conductivity by creating a smooth, uniform surface.
Drawbacks of Nickel Plating
Despite its advantages, nickel plating has some drawbacks:
- Increased Hardness: Nickel plating can make copper harder, which might cause inconsistencies in crimp terminations and affect the reliability of electrical connections.
Tin Plating on Copper
Tin plating is another popular method for enhancing copper properties, especially in electronic applications. This process is typically carried out through electroplating.
Electroplating Process
In tin electroplating, copper substrates are immersed in an electrolyte solution containing tin salts. An electric current is applied, causing tin ions to deposit onto the copper surface, forming a protective layer.
Benefits of Tin Plating
Tin plating offers the following benefits:
- Solderability: Tin plating greatly improves the solderability of copper components, making them more compatible with soldering processes used in electronics.
- Corrosion Protection: Tin provides moderate corrosion protection, sufficient for many electronic applications.
- Cost-Effectiveness: Tin plating is generally more cost-effective than nickel plating due to the lower cost of tin materials and simpler processing requirements.
Drawbacks of Tin Plating
Tin plating also has its limitations:
- Limited High-Temperature Use: Tin has a relatively low melting point and is not suitable for applications requiring high thermal stability.
- Whisker Growth: Tin plating can lead to the growth of tiny, hair-like structures called whiskers, which may cause electrical shorts and reliability issues in electronic components.
Comparison of Nickel and Tin Plating on Copper
| Feature | Nickel Plating | Tin Plating |
|---|---|---|
| Corrosion Resistance | High, suitable for harsh environments | Moderate, sufficient for typical electronic applications |
| Operating Temperature | Increases copper’s operating temperature | Limited high-temperature use due to tin’s melting point |
| Cost | Generally more expensive | More cost-effective due to lower material and process costs |
| Solderability | Not specifically enhanced for soldering | Highly beneficial for improving solderability |
| Hardness and Durability | Increases hardness, may affect crimp terminations | Does not significantly increase hardness |
Nickel plating is ideal for high corrosion resistance and elevated temperatures but is more expensive and can affect hardness. Tin plating is cost-effective, improves solderability, and is sufficient for most electronics but isn’t suitable for high temperatures and can lead to whisker growth.
Comparison of Corrosion Resistance
Nickel Plating on Copper
Corrosion Resistance
Nickel plating effectively enhances the corrosion resistance of copper. This is due to the formation of a dense, adherent nickel layer that acts as a barrier, preventing oxygen and moisture from reaching the copper substrate. The nickel layer is less reactive than copper, significantly reducing the rate of oxidation and corrosion. This makes nickel plating particularly effective in humid and moist environments, where copper alone would corrode more rapidly.
Chemical Resistance
Nickel’s chemical resistance further enhances its ability to protect copper from various corrosive agents, including acids and alkaline solutions, ensuring long-term durability and reliability. This property is crucial for applications where copper components are exposed to harsh chemicals, ensuring long-term durability and reliability.
Tin Plating on Copper
Corrosion Resistance
Tin plating also offers corrosion resistance by forming a protective layer over the copper. However, the effectiveness of tin plating can be compromised by its inherent porosity. Tiny pores in the tin layer can let corrosive agents reach the copper, causing localized corrosion. Additionally, tin is prone to whisker growth, which can create pathways for corrosion.
Oxidation Prevention
Though porous, tin plating sufficiently protects against oxidation in less demanding environments. The tin layer can prevent direct exposure of copper to atmospheric oxygen, thereby reducing the overall rate of corrosion. This makes tin plating suitable for many electronic applications where moderate corrosion resistance is sufficient.
Comparative Analysis of Corrosion Resistance
| Aspect | Nickel Plating | Tin Plating |
|---|---|---|
| Barrier Protection | Forms a dense, non-porous barrier that effectively blocks moisture and oxygen. | Provides a protective layer but can be porous, allowing some penetration of corrosive agents. |
| Chemical Resistance | High resistance to acids and alkalines, suitable for harsh chemical environments. | Moderate resistance, less effective in highly corrosive chemical environments. |
| Durability in Humid Conditions | Excellent performance, maintaining integrity in moist environments. | Good performance in moderate humidity but less effective in highly humid conditions. |
| Whisker Growth | No whisker growth, maintaining consistent protection. | Prone to whisker growth, which can compromise corrosion resistance. |
| Long-Term Protection | Provides long-lasting protection with minimal maintenance. | Offers adequate protection but may require more frequent inspection and maintenance. |
Nickel plating offers superior corrosion resistance compared to tin plating, particularly in harsh and humid environments. Its dense, non-porous layer and high chemical resistance make it ideal for applications requiring robust and long-term protection. Tin plating works well in many electronic applications but offers less corrosion resistance due to its porosity and whisker growth.
Mechanical Properties: Hardness, Wear Resistance, and Solderability
Nickel plating significantly boosts the hardness and wear resistance of copper substrates. Nickel’s inherent hardness helps it withstand mechanical stress, abrasion, and friction, making nickel-plated copper ideal for high-stress environments like mechanical components, connectors, and wear-resistant coatings. The high melting point of nickel (approximately 1455°C) also contributes to its structural integrity in high-temperature applications, ensuring long-lasting performance and durability.
Tin plating offers lower hardness and is softer and more ductile, which makes it suitable for applications needing flexibility and malleability, but less effective for wear resistance. Tin-plated copper is less suitable for high-wear applications but can be advantageous in processes that involve frequent bending or manipulation of the plated components.
Tin plating is excellent for soldering, making it a preferred choice in electronics. Its soft and ductile nature allows it to form reliable connections easily, creating strong joints in electronic components and PCBs. This property is particularly beneficial for efficient and reliable soldering, ensuring consistent performance in electronic assemblies.
Nickel plating is ideal for high hardness, wear resistance, and harsh environments, while tin plating excels in solderability and cost-effectiveness. The choice depends on the application’s specific needs.
| Property | Nickel Plating | Tin Plating |
|---|---|---|
| Hardness | High | Low |
| Wear Resistance | Excellent | Poor |
| Solderability | Poor | Excellent |
| High-Temperature Resistance | Excellent | Poor |
| Corrosion Resistance | Superior | Decent |
Solderability refers to the ease with which a material can be soldered to form a reliable electrical connection. High solderability ensures strong, durable joints and is critical in electronics manufacturing where consistent and reliable connections are essential for performance and longevity. Tin plating is particularly noted for its excellent solderability, making it a preferred choice in the electronics industry.
Electrical Conductivity and Its Impact on Electronic Applications
Electrical Conductivity Levels
Electrical conductivity measures how well a material can carry an electric current, which is crucial for the performance of electronic applications. When comparing nickel plating and tin plating on copper, their electrical conductivity levels are notably different.
Tin Plating Conductivity
Tin plating is recognized for its high electrical conductivity. This is attributed to tin’s low resistivity, approximately 115 nΩ*m, which ensures minimal energy loss during current flow. This characteristic makes tin plating particularly effective in applications where maintaining high conductivity is critical, such as in electrical components and connectors.
Nickel Plating Conductivity
Nickel plating, although conductive, has higher resistivity compared to tin. Electrolytic nickel plating has a resistivity of about 70 nΩm, whereas electroless nickel plating ranges from 550 to 925 nΩm. This higher resistivity means nickel is less efficient in purely electrical conductivity applications. However, nickel plating can still be advantageous in scenarios where its other properties, such as corrosion resistance and mechanical strength, are beneficial.
Impact on Electronic Applications
Tin Plating Advantages
Tin plating offers several advantages in electronic applications:
- Solderability: Tin plating is highly solderable due to its softness and ductility, which helps ensure reliable connections in electronic assemblies.
- Low-Temperature Applications: Tin plating is ideal for medium- to low-temperature environments (below 150°C), where it helps reduce contact resistance and maintain high electrical conductivity.
Nickel Plating Advantages
Nickel plating also provides significant benefits in specific electronic applications:
- High-Temperature Resistance: Nickel’s high melting point (around 1455°C) makes it suitable for high-temperature applications, ensuring long-term reliability and chemical stability.
- Mechanical Strength: Nickel’s hardness and mechanical strength are advantageous for components that endure high contact loads and pressures, such as switches and terminal pins.
Application Considerations
When choosing between nickel and tin plating for copper, consider the specific needs of your application:
- Corrosion Protection: Both nickel and tin plating offer corrosion protection, but their methods differ. Nickel acts as a barrier through cathodic protection, while tin’s effectiveness can vary with environmental conditions.
- Duplex Plating Systems: Combining nickel and tin in a duplex plating system can leverage the strengths of both materials, enhancing corrosion protection and improving solderability.
Tin plating excels in applications requiring high electrical conductivity and low-temperature environments, while nickel plating is ideal for high-temperature applications that demand mechanical strength and corrosion resistance.
High-Temperature Performance Differences
Nickel plating and tin plating have different melting points and thermal stability, affecting their use in high-temperature settings.
Nickel Plating
Nickel has a melting point of approximately 1455°C, much higher than tin’s. This high thermal stability makes nickel plating ideal for high-temperature applications, such as electrical connectors in industrial machinery. Nickel plating forms a dense, stable oxide layer that offers excellent corrosion resistance, protecting the copper substrate from oxygen and moisture even at high temperatures. Nickel plating enhances the hardness and wear resistance of copper substrates, making components more durable and able to withstand mechanical stress and friction. This durability is maintained even at high temperatures, ideal for industrial machinery or high-stress electrical connectors.
Tin Plating
Tin plating can provide good protection and solderability at lower temperatures, but it is susceptible to oxidation and degradation above 150°C. Although tin plating offers good corrosion resistance in chemical and humid environments, its effectiveness diminishes at elevated temperatures. Tin’s protective oxide layer can break down, leading to increased vulnerability to corrosion. Tin plating is softer and more ductile compared to nickel, which can be advantageous for soldering and joining processes. However, this softness means tin-plated components are less resistant to mechanical wear and abrasion. At high temperatures, the ductility of tin can lead to deformation, reducing the component’s effectiveness in applications requiring robust wear resistance.
Both nickel and tin plating impact the electrical conductivity of copper differently, especially under high-temperature conditions. Nickel plating maintains the electrical conductivity of copper, although nickel itself has higher resistivity compared to tin. While nickel plating does not significantly enhance conductivity, it ensures reliable performance in high-temperature environments where conductivity must be preserved. Tin plating can slightly increase the conductivity of copper surfaces due to tin’s lower resistivity. However, at high temperatures, the oxidation and degradation of tin can negatively affect conductivity.
The cost and environmental impact of nickel and tin plating vary, influencing their selection for high-temperature applications. Nickel plating is generally more expensive due to its superior durability and high-temperature performance. The higher initial cost can be offset by lower long-term maintenance expenses, as nickel-plated components tend to last longer in harsh environments. However, nickel plating poses environmental and health risks, requiring careful handling and disposal. Tin plating is typically more cost-effective, with lower material costs and simpler processing requirements. It is also safer and more environmentally friendly than nickel plating. While tin plating may not offer the same high-temperature performance, its lower cost and reduced environmental impact make it a viable option for applications with moderate thermal demands.
Benefits of Combined Nickel-Tin Duplex Plating Systems
Corrosion Resistance and Barrier Protection
Nickel Layer
Nickel plating forms a dense, smooth, and pore-free barrier on copper, offering exceptional corrosion resistance. This layer acts as a protective shield against corrosive agents, particularly in harsh or high-temperature environments. The nickel layer prevents copper diffusion, maintaining the integrity of subsequent coatings and electrical performance.
Tin Layer
Tin plating provides moderate corrosion resistance and, although less robust than nickel, its unique grain structure reduces overall porosity when layered on nickel. This dual-layer system improves the protective qualities, making the combined plating more effective in preventing corrosion.
Duplex Advantage
The nickel-tin duplex system creates a synergistic barrier that effectively limits corrosion pathways. The combination of a nickel underplate with a tin overplate enhances the protection of copper substrates by reducing porosity and extending the longevity of components in demanding applications.
Mechanical Strength and Wear Resistance
Nickel
Nickel plating is known for its high hardness, mechanical strength, and excellent wear resistance. This makes it suitable for components subjected to mechanical stress, abrasion, or elevated temperatures. Nickel enhances the durability and structural integrity of copper substrates, particularly in high-wear environments.
Tin
Tin plating provides a softer, more ductile surface, which is less wear-resistant but beneficial for applications requiring flexibility and ease of soldering. The softness of tin improves the interface during soldering, making it less brittle and more accommodating to thermal cycling.
Duplex Benefit
Using nickel as an underlayer adds significant mechanical strength and wear resistance to the system. The tin overlayer retains ductility and enhances solderability, creating an ideal balance for electrical components that need both durability and reliable solder joints.
Solderability and Electrical Performance
Tin
Tin is renowned for its excellent solderability due to its softness and ductility. This property facilitates easy and reliable solder joints, which are essential in electronic and electrical applications. Tin also provides good electrical conductivity, enhancing signal integrity and connection reliability.
Nickel
While nickel offers superior corrosion resistance, it is harder and less solderable than tin. Nickel is preferred in ultrasonic welding processes due to its compatibility but generally requires a tin overcoat for applications involving soldering.
Duplex Advantage
A nickel underplate combined with a tin overplate optimizes corrosion resistance and solderability. The nickel layer provides robust protection and wear resistance, while the tin layer ensures excellent solderability and electrical contact quality. This duplex system also limits the formation of intermetallic compounds at the copper interface, further improving reliability.
Thickness and Porosity Control
Using a duplex nickel-tin system can reduce the total plating thickness required for effective corrosion protection compared to single-layer plating. The differing grain structures of nickel and tin result in a less porous combined layer, which helps prevent moisture and chemical ingress. This approach enhances durability without significantly increasing coating thickness.
| Property | Nickel Plating | Tin Plating | Nickel-Tin Duplex Plating System |
|---|---|---|---|
| Corrosion Resistance | Excellent, especially in harsh/high-temperature conditions | Moderate, effective in mild/moderate environments | Superior barrier with reduced porosity, excellent in diverse conditions |
| Mechanical Strength | High hardness and wear resistance | Soft, ductile | High strength from nickel + ductility from tin |
| Solderability | Poor to moderate | Excellent | Excellent due to tin overlayer |
| Electrical Conductivity | Good but less than tin | Very good | Balanced; maintains conductivity and durability |
| Thickness Efficiency | Requires thicker, pore-free coating | Less critical for porosity | Reduced thickness due to complementary grain structures |
| Intermetallic Formation | Can form with solder, mitigated by tin layer | May promote intermetallics if directly on copper | Tin over nickel layer prevents intermetallics formation |
The combined nickel-tin duplex plating system effectively integrates the mechanical robustness and corrosion resistance of nickel with the superior solderability and electrical conductivity of tin. This system addresses the limitations of each metal when used alone, making it highly suitable for demanding industrial and electronic applications where both protection and reliable electrical performance are critical. The duplex plating not only enhances corrosion protection but also improves the longevity and functionality of copper components by optimizing coating thickness, reducing porosity, and preventing intermetallic formation.
Cost Considerations and Typical Industry Applications
Cost Considerations
When evaluating the cost implications of nickel and tin plating on copper, several factors must be considered, including material costs, processing complexity, and the intended application.
Nickel Plating Costs
Nickel plating typically costs more than tin plating for several reasons:
- Material Costs and Processing Complexity: Nickel is more expensive than tin and its plating process, particularly electroless nickel plating, is more complex and requires precise control, leading to higher overall costs.
- Durability and Longevity: Despite the higher initial cost, nickel plating offers superior corrosion resistance and durability, which can result in lower long-term maintenance and replacement costs.
Tin Plating Costs
Tin plating is typically more cost-effective due to:
- Lower Material Costs: Tin is less expensive than nickel, making the raw material cost for tin plating more affordable.
- Simpler Process: The tin plating process is generally less complex and requires less stringent control compared to nickel plating, reducing labor and operational costs.
- Sufficient Protection for Certain Applications: Tin plating offers good corrosion protection and solderability for electronics and consumer goods, making it a cost-effective choice for many industries.
Typical Industry Applications
The choice between nickel and tin plating on copper largely depends on the specific requirements of the application, including factors such as corrosion resistance, mechanical strength, and cost constraints.
Nickel Plating Applications
Nickel plating on copper is favored in industries where high durability and corrosion resistance are paramount:
- Electronics and Industrial Machinery: Nickel-plated copper is commonly used for connectors, circuit boards, and industrial parts such as pump shafts and hydraulic cylinders. The enhanced corrosion resistance and mechanical strength make it ideal for these applications.
- Automotive and Aerospace: In these sectors, components such as turbine blades, landing gear, and other critical parts benefit from nickel plating’s durability and ability to withstand harsh environmental conditions.
- Medical Devices: Nickel-plated copper is used in surgical instruments and implants due to its biocompatibility and resistance to corrosion, ensuring long-term reliability and safety in medical applications.
Tin Plating Applications
Tin plating is preferred in scenarios where cost-effectiveness and solderability are more critical:
- Electronics: Tin plating is widely used for soldering applications in the electronics industry. It ensures good solderability and provides a sufficient barrier against corrosion in less demanding environments.
- Consumer Goods: Tin plating is often used in consumer goods like household appliances and decorative items because it is affordable and provides basic corrosion protection.
Overall Pros and Cons of Each Plating Method
Nickel Plating on Copper
Pros
Corrosion Resistance and Durability: Nickel plating offers excellent corrosion resistance by creating a strong barrier against oxidation and moisture. This makes it ideal for applications in humid or moist environments, significantly extending the lifespan of copper components.
Hardness and Wear Resistance: Nickel is known for its high hardness, typically ranging from 150 to 700 Vickers. This attribute enhances the wear resistance of copper, making nickel-plated components suitable for mechanical applications that endure physical stress and abrasion.
Thermal and Electrical Conductivity: Nickel plating supports high thermal and electrical performance. Although copper is inherently conductive, the nickel layer protects the substrate without greatly impairing conductivity, making it suitable for high-performance electronic applications.
Aesthetic Appeal: Nickel plating imparts a bright, shiny finish to copper components, which remains visually appealing over time. This is particularly beneficial for consumer products and visible components.
Diffusion Barrier: Nickel acts as an effective barrier, preventing intermetallic formation between copper and tin if tin is used as an additional layer. This preserves solderability and prevents joint degradation in electronic applications.
Cons
Cost and Process Complexity: Electrolytic nickel plating is a costly, time-consuming process that requires strict environmental controls. Disposing of plating solutions can pose environmental challenges.
Non-Uniform Coating and Potential Cracking: Electrolytic processes may produce less uniform coatings that are prone to cracking and damage under stress or thermal cycling.
Solderability Challenges: Nickel surfaces oxidize over time, forming non-conductive oxide layers that reduce solderability. Specialized acid-activated fluxes are often required to remove oxides before soldering, which can leave corrosive residues.
Material Limitations: Nickel plating requires a conductive substrate, which limits its use to materials like copper.
Tin Plating on Copper
Pros
Solderability: Tin plating provides excellent and consistent solderability, especially with matte finishes. Its lower melting point allows for easier and more reliable soldering in electronic applications.
Corrosion Protection: Tin plating provides effective corrosion resistance, protecting copper from oxidation and environmental degradation. Although less robust than nickel, it is sufficient for many electronic applications.
Cost-Effective and Simpler Process: Tin plating is generally less expensive and faster to apply compared to nickel plating, making it attractive for large-scale or cost-sensitive applications.
Electrical Conductivity: Tin maintains good electrical conductivity and is compatible with common joining methods like soldering.
Cons
Formation of Intermetallic Compounds: Over time, tin can react with copper to form intermetallic layers, which reduce solderability and overall plating performance.
Lower Hardness and Wear Resistance: Tin is softer than nickel, offering less mechanical protection and durability under wear or abrasion.
Melting Point Limitation: Tin’s lower melting point limits its use in high-temperature environments or applications requiring high thermal stability.
Solderability Degradation: Although initially excellent, tin plating’s solderability can decline as oxides form, but this degradation is slower and less problematic than with nickel plating.
| Feature | Nickel Plating on Copper | Tin Plating on Copper |
|---|---|---|
| Corrosion Resistance | Excellent, robust barrier | Good, but less durable |
| Hardness & Wear Resistance | High hardness, superior durability | Softer, less wear resistant |
| Solderability | Challenging; requires acid flux to clean oxides | Excellent initially; consistent wetting |
| Intermetallic Formation | Acts as diffusion barrier, prevents intermetallics | Prone to intermetallic growth with copper |
| Thermal Stability | High melting point, stable at elevated temps | Lower melting point, limited high-temp use |
| Aesthetic Finish | Bright, long-lasting shine | Good, but may dull with time |
| Process Cost & Complexity | More expensive and time-consuming | More cost-effective and simpler |
| Environmental Impact | Higher, due to plating solution disposal | Lower, simpler waste management |
Nickel plating is ideal for applications needing high corrosion resistance, durability, thermal stability, and a premium finish, though it comes with higher costs and complexity. Tin plating is advantageous for its excellent solderability, cost efficiency, and adequate corrosion protection for general electronic applications, with limitations in mechanical strength and high-temperature performance.
Frequently Asked Questions
Below are answers to some frequently asked questions:
What are the key differences between nickel plating and tin plating on copper?
Nickel plating and tin plating on copper differ in several key aspects. Nickel plating significantly enhances corrosion resistance, hardness, and wear resistance, making it suitable for environments with high humidity and applications requiring durability, such as aerospace and electronics. It also maintains or slightly improves copper’s electrical conductivity and increases its operating temperature, which is beneficial for high thermal demand applications.
In contrast, tin plating is particularly valued for its excellent solderability and non-toxicity, making it ideal for food-grade applications and electronics where soldering is essential. While it offers good corrosion resistance, it does not enhance the thermal properties or hardness of copper to the same extent as nickel plating.
Cost-wise, nickel plating is generally more expensive and complex due to its higher durability and protective qualities, whereas tin plating is simpler and less costly. The choice between the two depends on the specific requirements of the application, balancing factors like corrosion resistance, mechanical properties, and cost.
Which plating is better for corrosion resistance on copper?
Nickel plating generally provides better corrosion resistance on copper compared to tin plating. This is due to nickel’s ability to form a dense, pore-free barrier that effectively prevents corrosive agents from reaching the copper substrate. Nickel’s higher hardness and mechanical strength also contribute to its superior performance in corrosive environments. On the other hand, tin plating offers corrosion protection through a different mechanism, which can be less effective depending on the environmental conditions. However, tin is more advantageous for applications requiring excellent solderability and ductility. For optimal corrosion resistance, especially in demanding applications, nickel plating is typically the preferred choice.
How do nickel and tin plating affect the electrical conductivity of copper?
Nickel and tin plating affect the electrical conductivity of copper differently. Tin plating generally enhances the electrical conductivity of copper by reducing contact resistance, making it suitable for medium- to low-temperature environments (below 150°C). However, tin itself has a higher electrical resistivity (approximately 115 nΩm) compared to nickel. When tin is plated over copper, it can form an intermetallic layer, potentially increasing resistance compared to bare or silver-plated copper.
Nickel plating, on the other hand, has a lower electrical resistivity (around 70 nΩm) than tin, which can make it more conductive in certain applications. Despite this, nickel is primarily valued for its high-temperature stability and excellent corrosion resistance rather than its conductivity. Therefore, while both plating methods can impact the electrical properties of copper, tin plating is typically chosen for its ability to reduce contact resistance in specific temperature ranges, whereas nickel plating is preferred for applications requiring durability in high-temperature environments.
Which plating method performs better at high temperatures?
For high-temperature applications, nickel plating performs better than tin plating on copper. Nickel plating offers superior thermal stability, with a high melting point of approximately 1455°C (2651°F), making it suitable for environments involving extreme heat. In contrast, tin plating has a much lower melting point of around 232°C (449.5°F), limiting its effectiveness in high-temperature conditions. Additionally, nickel plating provides excellent corrosion resistance and mechanical hardness, which are crucial for maintaining structural integrity and functionality under thermal stress. Therefore, for applications requiring durability and performance at elevated temperatures, nickel plating is the preferred choice.
What are the cost implications of choosing nickel plating versus tin plating?
When evaluating the cost implications of choosing nickel plating versus tin plating on copper, it is essential to consider both initial and long-term costs. Tin plating generally has lower initial costs due to simpler application processes and less expensive materials. This makes tin plating a cost-effective solution for applications where budget constraints are significant, such as in solderable coatings for electronic components.
In contrast, nickel plating, while more expensive upfront due to the complexity of the process and higher material costs, offers substantial long-term benefits. These include superior corrosion resistance, increased hardness, and enhanced durability. These properties can lead to lower maintenance and replacement costs over time, making nickel plating a more cost-effective choice for demanding applications in industries like aerospace, automotive, and high-performance electronics.
Can nickel and tin plating be combined for better performance?
Yes, combining nickel and tin plating can indeed provide better performance, especially when applied to copper substrates. This duplex system, which involves a nickel layer under a tin layer, leverages the strengths of both materials. Nickel offers excellent corrosion resistance, particularly in harsh environments, while tin provides superior solderability essential for reliable electrical connections. The nickel layer acts as a barrier, preventing the formation of intermetallic compounds between tin and copper, which can degrade solderability over time. Additionally, this combination reduces porosity, thereby enhancing the overall protective barrier against corrosion. This method is particularly beneficial for electronic applications, where both durability and solderability are critical.
