When it comes to protecting metal surfaces from the relentless assault of corrosion, two heavyweights often dominate the conversation: electroplating and galvanizing. Each method boasts its own set of advantages and quirks, but understanding the key differences can be a game-changer for industries ranging from automotive to construction. Have you ever wondered which technique offers better corrosion resistance or which is more cost-effective? This article will delve deep into the principles, processes, and practical applications of both electroplating and galvanizing. By the end, you’ll have a clearer picture of which method best suits your specific needs and why. Ready to uncover the secrets behind these essential metal coating techniques? Let’s dive in.
Introduction to Metal Coating
Metal coating involves applying a layer of metal onto the surface of a base material (substrate). This coating serves several purposes, including enhancing the material’s appearance, improving its resistance to corrosion, and increasing its
Corrosion naturally deteriorates metal over time due to environmental interactions like moisture, oxygen, and chemicals. Metal coatings act as a protective barrier, extending the lifespan of metal components and reducing maintenance costs.
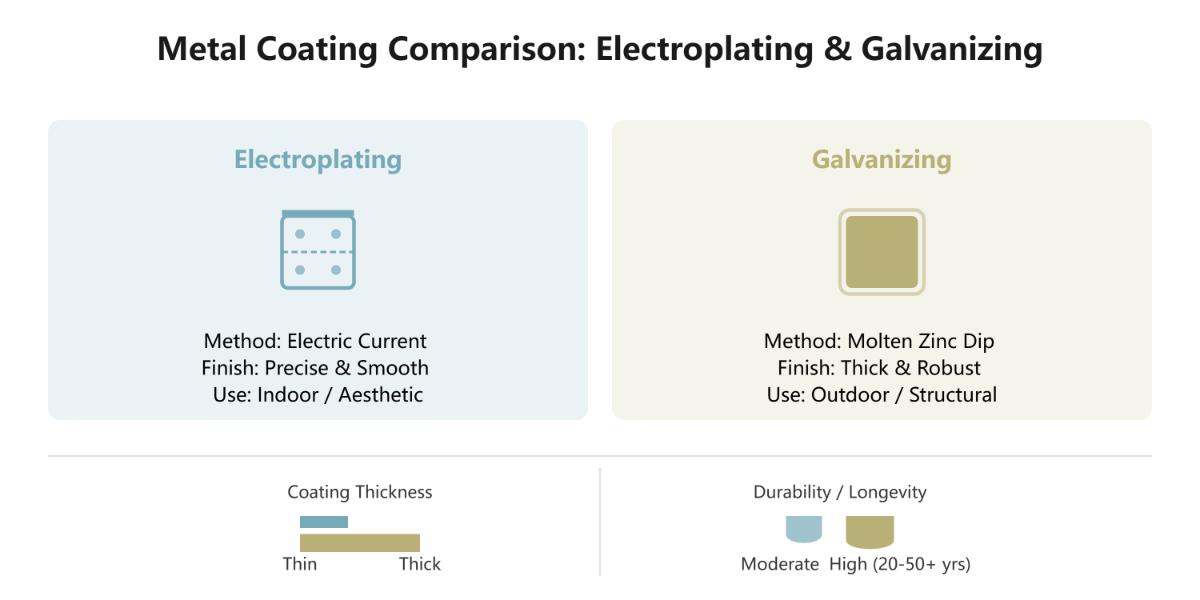
Various techniques are used to apply metal coatings, each suited to specific needs. Electroplating, for example, deposits a thin metal layer onto a substrate through an electrochemical process, using an electrolyte solution and electric current. This method is popular for both decorative and functional purposes, offering a smooth, attractive finish and is particularly effective for small, intricate parts.
Galvanizing, especially hot-dip galvanizing, involves immersing metal components in molten zinc, creating a strong bond and a thick, durable coating. This process is known for its superior corrosion resistance, making it ideal for outdoor structures and heavy-duty applications.
Electroplating typically results in a thinner coating, ranging from 5 to 12 microns. This thin layer is suitable for decorative finishes but may not provide sufficient protection in harsh environments. In contrast, galvanizing produces a much thicker coating, usually around 85 microns, offering enhanced durability and long-term protection.
While both methods improve corrosion resistance, galvanizing offers superior protection due to the thicker zinc layer. This makes galvanizing more suitable for applications exposed to aggressive environmental conditions. Electroplating, with its thinner layer, is better suited for less demanding environments and applications where aesthetics are a priority.
The cost of electroplating and galvanizing can vary depending on the application, material, and required coating thickness. Electroplating is generally more cost-effective for small, intricate parts and applications requiring a decorative finish. Galvanizing, although potentially more expensive, offers better long-term value for large structures and components exposed to harsh conditions.
Electroplating involves chemicals that can generate hazardous waste, requiring careful management. Galvanizing uses high temperatures and molten zinc, which also need responsible handling to minimize environmental impact. However, zinc is generally more environmentally friendly than the chemicals used in electroplating.
Sustainability is becoming increasingly important in material selection and processing. Galvanizing offers a self-healing property, where minor damages can repair themselves, contributing to its sustainability. Electroplating, while less durable, can be more sustainable for applications requiring frequent re-coating or where the base material can be reused.
Understanding these differences helps in selecting the appropriate metal coating technique based on the specific requirements of the application.
Understanding Electroplating
History and Development
Electroplating has a storied history dating back to the early 19th century. Italian chemist Luigi Brugnatelli first discovered the process in 1805, using a voltaic pile to deposit gold onto a substrate. This groundbreaking discovery laid the foundation for modern electroplating techniques. Over time, advancements in chemistry and electrical engineering have refined the process, enhancing precision and expanding its applications across various industries.
Principles and Processes
Electroplating involves depositing a metal layer onto a substrate through an electrochemical process. An electric current is used to convert dissolved metal ions into a solid metal layer on the surface of the workpiece. The basic components of an electroplating system include the anode, cathode, electrolyte solution, and a power source.
Key Steps in Electroplating
- Preparation of the Substrate: The substrate or workpiece is thoroughly cleaned to remove any dirt, grease, or oxidation. This ensures proper adhesion of the metal coating.
- Electrolyte Solution Preparation: The electrolyte solution contains metal salts and other chemicals that facilitate the plating process.
- Setup of Electroplating Cell: The anode, which is the metal to be plated, and the cathode, which is the workpiece, are placed in the electrolyte solution. The power source then supplies the current needed for the plating process.
- Electrodeposition: When the current is applied, metal ions from the electrolyte solution are reduced and deposited onto the cathode, forming a thin, uniform layer.
Advantages and Disadvantages
Electroplating offers several benefits, but it also has some limitations. Understanding these can help in selecting the right coating method for specific applications.
Advantages
- Improved Corrosion Resistance: Electroplating enhances the substrate’s resistance to corrosion, extending its lifespan.
- Enhanced Aesthetic Appeal: Electroplating can create a bright, shiny finish, perfect for enhancing the look of decorative items.
- Increased Electrical Conductivity: Electroplated coatings, particularly those with metals like gold and silver, improve the electrical conductivity of components.
- Versatility: Electroplating can be applied to a wide range of materials and shapes, including complex geometries and small parts.
Disadvantages
- Environmental Concerns: The process involves the use of hazardous chemicals, which require proper handling and disposal to mitigate environmental impact.
- Cost: The cost of electroplating can be higher than other coating methods, particularly when used for large-scale projects.
- Thickness Limitations: The coatings are generally thin, which may not provide sufficient protection in highly corrosive environments.
Typical Applications and Industry Use Cases
Electroplating is widely used across various industries due to its versatility and effectiveness in enhancing material properties.
Automotive Industry
In the automotive sector, electroplating is used to improve the durability and appearance of components such as bumpers, rims, and various engine parts. The process provides a protective layer that resists wear and corrosion, contributing to the longevity of the vehicle.
Electronics
Electroplating is critical in the electronics industry, where it is used to coat connectors, circuit boards, and other components. The process ensures excellent electrical conductivity and provides a reliable connection, which is essential for the performance of electronic devices.
Jewelry and Decorative Items
The aesthetic appeal of electroplated items makes the process popular in the production of jewelry and decorative items. Metals like gold, silver, and platinum are commonly used to give a high-quality finish that enhances the visual appeal of these products.
Aerospace
In aerospace, electroplating protects components that face harsh conditions, ensuring they remain durable and reliable. The process helps in maintaining the integrity and performance of critical parts, such as turbine blades and landing gear.
Understanding Galvanizing
History and Development
Galvanizing has a rich history that dates back to the early 19th century. The process was first observed by French engineer Stanislas Sorel, who in 1836 filed a patent for the method of coating iron with zinc to prevent rusting. Over the years, the technique has evolved significantly, incorporating advancements in metallurgical science and industrial practices to enhance its effectiveness and application scope.
Principles and Processes
Galvanizing primarily involves coating steel or iron with a layer of zinc to protect it from corrosion. The most common and effective method is hot-dip galvanizing.
Hot-Dip Galvanizing Process
- Surface Preparation: The metal surface is cleaned to remove impurities, such as grease, dirt, and rust, through processes like degreasing, pickling, and fluxing. This step is crucial for ensuring proper adhesion of the zinc coating.
- Immersion in Molten Zinc: The cleaned metal is then immersed in a bath of molten zinc, typically maintained at around 450°C (842°F). During this immersion, a metallurgical reaction occurs, forming a series of zinc-iron alloy layers on the surface of the steel or iron.
- Cooling and Inspection: After the metal is removed from the zinc bath, it is cooled, usually by air or water quenching. The coated metal is then inspected for coating thickness, adhesion, and
Advantages and Disadvantages
Advantages
Galvanizing offers several significant benefits, making it a preferred choice for many applications.
- Excellent Corrosion Resistance: The zinc coating provides a robust barrier against moisture and oxygen, significantly enhancing the metal’s resistance to rust and corrosion.
- Durability: Galvanized coatings are known for their longevity, often lasting decades even in harsh environments.
- Self-Healing Properties: Zinc has the ability to protect exposed areas of the base metal through a process called galvanic or sacrificial protection. If the coating is scratched, the zinc will corrode in place of the steel, protecting it from rust.
- Low Maintenance: Galvanized structures require minimal maintenance, reducing long-term costs associated with upkeep and repairs.
Disadvantages
- Initial Cost: The initial cost of galvanizing can be higher compared to other coating methods, particularly for large-scale projects.
- Appearance: The finish of galvanized coatings can be less aesthetically pleasing compared to electroplated finishes, which might not be suitable for applications where appearance is critical.
- Thickness Control: While the thick coating is advantageous for durability, it can be challenging to control thickness precisely, which may not be ideal for parts requiring high precision.
Typical Applications and Industry Use Cases
Galvanizing is widely used across various industries due to its superior corrosion protection and durability.
Construction
In the construction industry, galvanized steel is extensively used for structural components, such as beams, columns, and bridges. Its ability to withstand harsh environmental conditions makes it ideal for outdoor and heavy-duty applications.
Automotive
The automotive sector utilizes galvanized steel for manufacturing car bodies and frames, providing enhanced resistance to rust and extending the lifespan of vehicles. This is particularly important for vehicles exposed to road salts and moisture.
Agriculture
Galvanized steel is a preferred material for agricultural structures, including silos, fencing, and irrigation systems. Its durability and low maintenance requirements make it suitable for the demanding conditions of agricultural environments.
Utilities and Infrastructure
Utilities and infrastructure projects benefit from galvanized components like transmission towers, pipelines, and street lighting poles. The long-lasting protection ensures reliability and reduces the need for frequent replacements.
Key Aspects of Galvanizing
- Coating Thickness: Hot-dip galvanizing typically results in a thicker coating compared to other methods, often around 85 microns, providing robust protection.
- Process Simplicity: The hot-dip process is straightforward, involving immersion in molten zinc, which creates a strong metallurgical bond.
- Comprehensive Coverage: It ensures complete coverage, including interior surfaces of hollow structures, which is crucial for preventing internal corrosion.
Comparative Analysis: Electroplating vs Galvanizing
Electroplating
Process Description
Electroplating uses an electrochemical process to coat a metal layer onto a substrate. This technique employs an electric current to reduce dissolved metal cations in an electrolyte solution, resulting in a coherent metal coating on the substrate. Common metals used in electroplating include zinc, nickel, chromium, and gold.
Advantages
- Aesthetic Appeal: Electroplating can achieve a highly polished, decorative finish, making it ideal for items where appearance is critical.
- Precision Control: The coating thickness can be precisely controlled by adjusting the current, voltage, and plating time.
- Versatility: Electroplating can be applied to a wide range of metals and can produce various finishes, including shiny, matte, and textured surfaces.
- Improved Electrical Conductivity: Metals like gold and silver used in electroplating enhance the electrical conductivity of electronic components.
Disadvantages
- Thin Coating: The coatings produced are typically thin (5-12 microns), which may not provide sufficient protection in highly corrosive environments.
- Environmental Concerns: The process involves hazardous chemicals that need careful handling and disposal, posing environmental risks.
- Cost: Electroplating can be more costly, especially for larger components or when precious metals are used.
Galvanizing
Process Description
Galvanizing, particularly hot-dip galvanizing, involves immersing steel or iron in a bath of molten zinc. The zinc reacts with the base metal to form a series of zinc-iron alloy layers, topped by a layer of pure zinc. This process creates a robust, protective coating that is significantly thicker than electroplated layers.
Advantages
- Thick Coating: The resulting coating from hot-dip galvanizing is much thicker, often up to 85 microns, providing superior durability and long-lasting protection.
- Excellent Corrosion Resistance: The thick zinc coating offers strong protection against corrosion, making it ideal for harsh environments.
- Self-Healing Properties: If damaged, the zinc coating can self-heal, protecting the metal underneath.
- Cost-Effective for Large Projects: Though initial costs are higher, lower maintenance makes it economical for large-scale projects.
Disadvantages
- Initial Cost: The upfront costs can be higher, particularly for small or intricately shaped parts.
- Surface Finish: The finish is typically dull and rough, often needing extra treatments for a better appearance.
- Thickness Control: It can be challenging to control the coating thickness precisely, which may not be suitable for parts requiring high precision.
Key Differences
| Feature | Electroplating | Galvanizing (Hot-Dip) |
|---|---|---|
| Application Method | Electrolysis in a metal ion solution. | Immersion in molten zinc. |
| Coating Thickness | Thin (5-12 microns) | Thick (up to 85 microns) |
| Corrosion Resistance | Limited, for mild environments. | High, suitable for harsh environments. |
| Application Suitability | Small parts, decorative finishes. | Large structures, structural applications. |
| Cost and Maintenance | Lower initial cost, higher maintenance. | Higher initial cost, lower maintenance. |
Application Suitability
The suitability of electroplating and galvanizing depends on the specific needs of the application:
- Electroplating is ideal for small, intricate parts where a decorative finish is desired, such as in jewelry, electronics, and automotive components.
- Galvanizing is better suited for large-scale structural applications exposed to harsh environmental conditions, such as in construction, automotive frames, and agricultural equipment.
Cost Analysis
While electroplating may offer lower initial costs for small items, galvanizing provides better long-term value for larger projects due to its durability and low maintenance requirements. The choice between these methods should consider both the upfront costs and the long-term benefits associated with each process.
Environmental Impact
Electroplating involves the use of hazardous chemicals that require careful disposal and handling to mitigate environmental harm. In contrast, galvanizing, particularly with zinc, poses fewer environmental risks, although the process still requires responsible management to minimize its ecological footprint.
Sustainability Considerations
Galvanizing offers a self-healing property, where minor damages can repair themselves, contributing to its sustainability. Electroplating, while less durable, can be more sustainable for applications requiring frequent re-coating or where the base material can be reused.
Selecting the Right Method for Your Application
When choosing the best metal coating method for your application, consider several factors to ensure optimal performance and cost-effectiveness.
Material and Environment
The base material and environmental conditions jointly influence the choice of coating method. Electroplating is suitable for a wide range of materials, including steel, brass, copper, and aluminum, particularly for precision parts requiring a thin, uniform coating. In contrast, galvanizing is predominantly used for steel and iron due to the metallurgical bond formed between zinc and these metals. Electroplating is ideal for indoor applications or environments with minimal exposure to corrosive elements, providing adequate protection for decorative items, electronic components, and automotive parts that do not face extreme conditions. Galvanizing offers superior protection in harsh environments, such as outdoor structures, marine applications, and industrial settings, where the thicker zinc coating can withstand severe corrosion.
Cost
Electroplating often costs less initially, making it ideal for smaller, decorative items. However, the thinner coating may necessitate more frequent maintenance and re-coating, potentially increasing long-term costs. Galvanizing, while having a higher upfront cost, provides extended durability and requires less maintenance over time, resulting in cost savings for large-scale and structural applications.
Use Cases in Different Industries
Automotive
In the automotive industry, both electroplating and galvanizing are used, depending on the component and its requirements. Electroplating is preferred for smaller parts, such as fasteners, decorative trims, and connectors, where a smooth finish and precise coating are essential. Galvanizing is used for larger structural components, like car bodies and frames, which require robust corrosion resistance to ensure safety and longevity.
Construction
Construction applications often favor galvanizing due to the need for long-lasting protection in exposed environments. Steel beams, columns, and other structural elements are commonly hot-dip galvanized to prevent rust and degradation over decades. Electroplating is less common in construction but may be used for smaller, decorative elements or components requiring a polished finish.
Electronics
The electronics industry relies heavily on electroplating to enhance the conductivity and corrosion resistance of connectors, circuit boards, and other critical components. Metals like gold, silver, and nickel are frequently electroplated onto copper or aluminum substrates to improve performance and reliability. Galvanizing is rarely used in electronics due to the thickness of the coating and the specific material requirements.
Expert Recommendations
When deciding between electroplating and galvanizing, it is essential to consult with industry experts who can provide tailored advice based on the specific application requirements. Factors such as the expected lifespan, environmental exposure, and budget constraints will guide the selection process.
Balancing Performance and Aesthetics
In applications where both performance and aesthetics are important, a hybrid approach may be considered. For instance, a component could be galvanized for structural integrity and then electroplated with a thin layer of a decorative metal to achieve the desired appearance. This combination leverages the strengths of both methods, providing comprehensive protection and an attractive finish.
Carefully evaluating these factors will help you choose the best metal coating method for your application, ensuring it performs well, lasts long, and is cost-effective.
New Technologies in Metal Coating
Recent Advances in Electroplating
Pulse Electroplating
Pulse electroplating enhances metal coatings by using intermittent electrical currents, alternating between on and off cycles, unlike traditional methods with continuous current. This technique reduces dendrite formation, improves coating uniformity, and allows better control over the microstructure of the deposited metal. It is especially beneficial for applications requiring high precision and fine detail, such as in the electronics and aerospace industries.
Nanostructured Coatings
The development of nanostructured coatings has significantly improved metal coating performance. By incorporating nanoparticles, these coatings achieve enhanced hardness, wear resistance, and corrosion protection, making them ideal for demanding environments where traditional coatings would fail. These nanocomposite coatings offer new possibilities for extending the lifespan and functionality of components.
Innovations in Galvanizing
Thermal Diffusion Galvanizing
Thermal diffusion galvanizing, or Sherardizing, involves heating steel parts with zinc powder in a closed container at temperatures below zinc’s melting point. This process causes the zinc to vaporize and diffuse into the steel’s surface, forming a zinc-iron alloy coating. It provides uniform coverage, even on complex geometries, and offers excellent corrosion resistance, making it particularly suitable for small parts and fasteners.
Zinc-Aluminum Coatings
Recent galvanizing advancements have developed zinc-aluminum coatings, which combine the benefits of both metals. These coatings, often referred to as Galfan, consist of a zinc-aluminum alloy that provides superior corrosion resistance compared to pure zinc coatings. The addition of aluminum enhances the coating’s ability to withstand harsh environments, making it ideal for marine and industrial applications. Zinc-aluminum coatings also offer better adhesion and flexibility, reducing the risk of cracking and peeling.
Future Trends and Developments
Eco-Friendly Processes
As environmental concerns grow, the metal coating industry is moving towards more sustainable practices. Innovations in electroplating and galvanizing focus on reducing the use of hazardous chemicals and minimizing waste. New electroplating baths are being developed with less toxic substances, and advancements in recycling techniques help reclaim and reuse plating materials. Similarly, the galvanizing industry explores ways to reduce energy consumption and improve zinc usage efficiency, making the process more environmentally friendly.
Smart Coatings
Smart coatings that respond to environmental changes represent the future of metal protection. These coatings provide real-time feedback on their condition, such as detecting corrosion or wear, and can even self-heal minor damages. Smart coatings incorporate advanced materials and sensors that monitor the integrity of the coating and the underlying metal, offering valuable data for maintenance and performance optimization. This technology holds great promise for critical infrastructure, where early detection of potential issues can prevent catastrophic failures.
High-Performance Alloys
Research into high-performance alloy coatings is opening new frontiers in metal protection. Alloys combining multiple metals in specific proportions offer tailored properties, such as enhanced thermal stability, superior mechanical strength, and exceptional corrosion resistance. These high-performance alloys are being developed for specialized applications, including aerospace, defense, and energy sectors, where traditional coatings may not meet stringent performance requirements.
Case Studies and Industry Expert Insights
Automotive Industry
In the automotive industry, both electroplating and galvanizing are utilized extensively, but in different applications. Car manufacturers often use electroplating for components like bumpers, trim pieces, and fasteners. These components need a smooth, decorative finish along with enhanced corrosion resistance. Electroplating with chrome or nickel provides the necessary aesthetic appeal and protection against minor environmental exposure.
On the other hand, galvanizing is used for more substantial automotive parts such as chassis and structural components. These parts need robust protection against corrosion due to their exposure to road salts, moisture, and other harsh conditions. Hot-dip galvanizing ensures these components remain durable and resistant to rust, extending the vehicle’s lifespan and maintaining safety standards.
Construction Sector
In the construction sector, galvanizing is clearly preferred, especially for structural applications. Steel beams, columns, and frameworks used in building construction are often hot-dip galvanized to protect them from corrosion. This is particularly crucial for outdoor structures like bridges and high-rise buildings that face constant exposure to the elements. The thick zinc coating provided by galvanizing ensures long-term durability and reduced maintenance costs.
Electroplating, although less common in structural applications, is sometimes used for smaller, decorative elements within buildings. Items such as door handles, fixtures, and ornamental hardware benefit from electroplating due to its ability to produce a visually appealing finish while providing adequate corrosion resistance for indoor environments.
Electronics and Telecommunications
Electroplating is crucial in the electronics and telecommunications industries for enhancing the performance and longevity of components. Connectors, circuit boards, and various electronic parts are often electroplated with metals like gold, silver, and nickel to improve electrical conductivity and resistance to oxidation. This ensures reliable performance and connectivity in electronic devices.
Galvanizing is less prevalent in electronics due to the thicker coatings and the specific material requirements of electronic components. However, it may be used in certain telecommunications infrastructure, such as towers and outdoor enclosures, where corrosion resistance is paramount.
Expert Opinions and Recommendations
Industry experts highlight the superior durability of galvanized coatings in environments with high corrosion potential. For instance, hot-dip galvanizing is recommended in marine and coastal applications because it can withstand saltwater exposure and offers long-term protection. Experts advise that while electroplating can offer excellent corrosion resistance in less demanding environments, its thinner coatings may not be sufficient for harsh conditions.
From a cost perspective, experts note that while electroplating can be more economical for small-scale applications requiring precise and decorative finishes, galvanizing offers better long-term value for large-scale projects. The initial higher cost of galvanizing is offset by its reduced maintenance needs and extended lifespan, making it a cost-effective choice for infrastructure and industrial applications.
Environmental experts emphasize the importance of responsible handling and disposal of chemicals used in electroplating. This process involves hazardous substances that could pose environmental risks if not properly managed. Conversely, galvanizing, while still requiring careful management, generally poses fewer environmental concerns, particularly with advancements aimed at reducing energy consumption and improving zinc usage efficiency.
Experts recommend selecting the coating method based on specific application needs. For decorative items and components requiring high precision, electroplating is advised due to its ability to produce a smooth, uniform finish. For structural elements and parts exposed to severe environmental conditions, galvanizing is preferred due to its thicker, more durable coating.
Frequently Asked Questions
Below are answers to some frequently asked questions:
What are the differences between electroplating and galvanizing?
Electroplating and galvanizing are both metal coating processes used to enhance corrosion resistance and durability, but they differ significantly in their methods and applications.
Electroplating involves immersing the substrate in an electrolytic solution and using an electric current to deposit metal ions onto the surface. This process allows for precise control over coating thickness and can apply various metals, making it ideal for small, intricate parts that require a smooth finish. However, the resulting coating is typically thinner and less durable compared to galvanizing, making it better suited for aesthetic or indoor applications.
Galvanizing, particularly hot-dip galvanizing, involves submerging steel into a bath of molten zinc. This creates a much thicker coating that provides superior corrosion resistance and durability, often lasting 20 to 50 years depending on environmental conditions. Galvanizing is particularly effective for large structural components exposed to harsh outdoor environments, such as bridges or construction materials.
Which method is more effective for corrosion resistance?
When comparing electroplating and galvanizing for corrosion resistance, galvanizing is generally more effective. Galvanizing, particularly hot-dip galvanizing, produces a thicker zinc coating (50 to 150 microns) that provides both a strong physical barrier and cathodic protection. This means that even if the coating is damaged, the zinc continues to protect the underlying metal. Therefore, galvanizing is ideal for large structures exposed to harsh environments, offering long-term durability and robust protection.
Electroplating, on the other hand, deposits a thinner zinc layer (5 to 25 microns) through an electrolytic process. While this method is suitable for precision parts requiring a smooth finish, it does not offer the same level of cathodic protection and is more susceptible to mechanical damage. Consequently, electroplating is better suited for applications with less demanding corrosion resistance requirements, such as automotive and electronic components.
How do the costs of electroplating and galvanizing compare?
Electroplating and galvanizing differ significantly in terms of cost. Electroplating, particularly zinc plating, is generally less expensive initially due to its less resource-intensive electrolytic process. This method is suitable for applications requiring a smooth finish and is cost-effective for mass production, though it offers a thinner coating and requires more frequent maintenance.
On the other hand, galvanizing, especially hot-dip galvanizing, involves immersing steel in a molten zinc bath, resulting in a thicker, more durable coating. While the initial cost is higher, galvanizing provides long-term savings through superior durability and minimal maintenance needs. This makes it ideal for heavy-duty structural applications exposed to harsh conditions.
What are the environmental impacts of each method?
Electroplating and galvanizing both have notable environmental impacts, primarily due to their processes and the chemicals involved. Electroplating uses hazardous chemicals like cyanide and heavy metals, which can contaminate water and soil if not properly managed. The process also generates significant wastewater containing toxic substances, necessitating advanced treatment systems to mitigate pollution. Additionally, electroplating is energy-intensive, contributing to greenhouse gas emissions, especially when fossil fuels are the energy source.
In contrast, galvanizing involves fewer hazardous chemicals but requires high temperatures, making it energy-intensive and contributing to greenhouse gas emissions. While it generates less wastewater than electroplating, improper waste management can still lead to environmental issues. However, galvanizing offers environmental benefits such as the durability of the coating, which reduces the need for frequent replacements, thus minimizing resource consumption and waste generation over the long term.
In which industries are electroplating and galvanizing most commonly used?
Electroplating is commonly used in the automotive, aerospace, electronics, and jewelry industries. In the automotive sector, it is applied to small parts like screws and bolts for both functional and decorative purposes. The aerospace industry utilizes electroplating to protect components from harsh environments. In electronics, it is crucial for depositing conductors in printed circuit boards and integrated circuits. Additionally, electroplating enhances the visual appeal of jewelry and household fixtures.
Galvanizing, on the other hand, is extensively used in the construction and infrastructure sectors due to its superior corrosion resistance. It is also prevalent in industrial and agricultural settings for equipment and structures exposed to harsh conditions. Furthermore, the automotive industry employs hot dip galvanizing for large parts like chassis and engine components that require high durability.
What are the latest advancements in metal coating technologies?
Recent advancements in metal coating technologies have significantly improved the durability, sustainability, and functionality of both electroplating and galvanizing processes. Innovations such as nanocoatings and multilayer systems enhance corrosion resistance and durability by manipulating coatings at the nanoscale. Atomic Layer Deposition (ALD) offers precise control over coating thickness, beneficial for complex geometries and microelectronics. Plasma-Enhanced Chemical Vapor Deposition (PECVD) improves surface properties through enhanced adhesion and reactivity using plasma energy. Techniques like laser direct structuring (LDS) and autocatalytic (electroless) plating have advanced the selective metal plating on polymers, enhancing the functionalization of non-conductive surfaces. Additionally, there is a growing emphasis on sustainable coating solutions, including water-based and low VOC options, which align with eco-friendly practices without compromising performance. These advancements ensure that both electroplating and galvanizing continue to evolve and meet the diverse needs of various industries.
