In the world of medical imaging, the question of whether a material is compatible with Magnetic Resonance Imaging (MRI) is crucial. Stainless steel, a common material in medical implants, often raises concerns about its MRI compatibility. Unlike Nickel – Titanium (NiTi) alloys, which have distinct MRI – related properties, stainless steel’s behavior in MRI environments isn’t always straightforward.
This article will delve into the fundamentals of MRI compatibility, explore the properties of both stainless steel and NiTi alloys, and conduct a comparative analysis. So, how do these materials stack up in terms of MRI artifacts and patient safety? Let’s find out.
Understanding MRI and Compatibility Fundamentals
What is Magnetic Resonance Imaging (MRI)?
Definition and basic principles of MRI
Magnetic Resonance Imaging (MRI) is a medical imaging technique that provides detailed images of the body’s internal structures without being invasive. Unlike X – rays or CT scans, which use ionizing radiation, MRI uses a strong magnetic field and radio waves to generate these images. This makes it a safer option for many patients, especially when multiple scans are required.
How MRI works: the role of magnetic fields and radio waves
In an MRI scanner, the patient lies inside a large magnet. The magnetic field aligns the hydrogen nuclei in the body’s water molecules. When radio waves are applied, these nuclei absorb the energy and move. As they return to their original state, they emit signals that are used to create images.
MRI Compatibility: Key Concepts
MRI compatibility refers to the safety and functionality of materials or devices in the MRI environment. Compatible materials should not interfere with imaging or pose risks due to the strong magnetic field. This is crucial for patients with implanted devices like pacemakers or stents.
Standards and guidelines for MRI compatibility
Regulatory bodies like the FDA require manufacturers to test their devices for MRI compatibility, checking for magnetic interactions, heating effects, and image artifacts. Products that pass these tests are labeled as MRI – safe or MRI – conditional, indicating they are suitable for use in MRI environments.
Stainless Steel in the MRI Context
Properties of Stainless Steel
Stainless steel is an alloy made primarily of iron, chromium, and nickel, along with other elements. This composition offers several advantages, including high corrosion resistance, excellent mechanical strength, and biocompatibility. These characteristics make stainless steel a popular choice for various medical implants and devices, such as surgical instruments, orthopedic implants, and cardiovascular stents.
Composition and Characteristics
Types 304 and 316L are commonly used stainless steel alloys in medical applications. Type 304 stainless steel contains approximately 18% chromium and 8% nickel, while type 316L includes additional molybdenum, which enhances its corrosion resistance, especially in saline environments. The “L” in 316L means “low carbon,” preventing carbide formation during welding and preserving corrosion resistance.
Common Uses in Medical Implants and Devices
Stainless steel is extensively used in the medical field due to its favorable properties. Common applications include:
- Orthopedic Implants: Such as screws, plates, and rods for fracture fixation.
- Cardiovascular Devices: Including stents and heart valve components.
- Surgical Instruments: Scalpels, forceps, and retractors are frequently made from stainless steel due to its durability and ease of sterilization.
MRI Compatibility of Stainless Steel
Behavior of Stainless Steel in MRI Environments
When exposed to the magnetic fields in an MRI scanner, stainless steel can exhibit ferromagnetic behavior, particularly in alloys containing higher iron and nickel content. This ferromagnetism can cause significant challenges in MRI settings, leading to image artifacts and potential safety concerns.
Potential MRI Artifacts and Safety Concerns
Stainless steel’s ferromagnetic properties can result in artifacts during MRI scans. These artifacts are distortions or voids in the MRI images, which occur due to local magnetic field disruptions caused by the stainless steel implant. The severity of these artifacts depends on the type of MRI sequence used, with gradient-echo sequences being particularly susceptible.
In terms of safety, stainless steel implants generally do not pose significant risks within MRI environments up to 3 Tesla. However, minor interactions such as translational attraction and rotational torque may occur, which can cause discomfort or minor movement of the implant. Heating effects are usually minimal but should still be considered, especially in longer MRI procedures.
Regulatory Guidelines for Using Stainless Steel in MRI
Regulatory bodies like the FDA classify stainless steel implants by their MRI compatibility. Stainless steel implants are often labeled as “MRI conditional,” indicating that they can be used safely under specific conditions. These conditions typically include limitations on the magnetic field strength (e.g., up to 3 Tesla) and specific guidelines for the positioning and duration of the MRI scan to minimize potential risks.
Specific Applications and Considerations
- Stainless Steel Sutures: Recent studies have shown that stainless steel sutures used in reconstructive surgeries, such as microtia reconstruction, are safe in MRI environments up to 3 Tesla. These sutures exhibit minimal magnetic interactions and produce only minor artifacts, making them suitable for use in patients requiring MRI scans.
- MRI Conditional Status: Stainless steel implants that are classified as MRI conditional must be used according to specific guidelines to ensure safety. This includes adhering to field strength limitations and avoiding prolonged exposure to the magnetic field to prevent heating and movement risks.
Stainless steel, while generally safe and widely used in medical applications, presents certain challenges in MRI environments due to its ferromagnetic properties and potential for artifact generation. Understanding and adhering to regulatory guidelines and specific application considerations are crucial for ensuring patient safety and optimal imaging outcomes.
Nickel – Titanium (NiTi) Alloys in MRI
Properties of NiTi Alloys
Nickel-Titanium (NiTi) alloys, commonly known as nitinol, possess unique characteristics that make them highly suitable for medical applications, especially in the context of MRI. These alloys are composed primarily of nickel and titanium, and are renowned for their shape memory and superelastic properties.
Composition and Unique Characteristics
NiTi alloys are particularly known for their shape memory and superelastic properties, which make them valuable in medical applications. These properties are particularly beneficial in medical implants, providing both flexibility and durability.
- Shape Memory Effect: The shape memory effect allows these devices to be delivered in a compact form and then expand to the desired shape within the body.
- Superelasticity: This property enables the material to undergo significant deformation and return to its original shape without permanent damage, making it ideal for dynamic environments like the cardiovascular system.
Common Applications in the Medical Field
NiTi alloys are extensively used in various medical devices due to their favorable properties. These applications include:
- Stents: NiTi stents are widely used in cardiovascular interventions due to their ability to conform to the vessel’s shape and maintain patency.
- Orthopedic Implants: Such as bone staples and fixation devices, where flexibility and strength are crucial.
- Guidewires: Utilized in minimally invasive surgeries to navigate through vessels and pathways.
MRI Compatibility of NiTi Alloys
Behavior of NiTi Alloys in MRI Environments
One of the key advantages of NiTi alloys in MRI settings is their non-ferromagnetic nature. Unlike stainless steel, NiTi does not interact strongly with the magnetic fields generated by MRI machines, which results in fewer complications during imaging procedures.
MRI Artifacts and Safety Profile of NiTi Alloys
NiTi alloys produce minimal artifacts during MRI scans, which is crucial for obtaining accurate images. The non-ferromagnetic properties of NiTi alloys ensure that they do not significantly distort the magnetic field, leading to clearer images with minimal signal loss.
- Artifact Minimization: NiTi alloys generate negligible artifacts, allowing for better visualization of the stent lumen and surrounding tissues. This is particularly beneficial in diagnostic imaging where clarity is paramount.
- Safety: NiTi stents are safe for use in MRI environments, as they are not prone to dislodgment or significant heating effects. This ensures patient safety during MRI procedures.
Regulatory Guidelines for Using NiTi Alloys in MRI
Regulatory bodies have established guidelines to ensure the safe use of NiTi alloys in MRI environments. These guidelines classify NiTi implants as “MRI safe” or “MRI conditional,” indicating their suitability under specific conditions such as limitations on field strength and exposure duration.
Adherence to these guidelines is essential for ensuring patient safety and optimal imaging outcomes when using NiTi implants in MRI settings.
Comparative Analysis: Stainless Steel vs. NiTi Alloys in MRI
MRI Artifacts and Image Quality
MRI artifacts and image quality are significant considerations when evaluating materials for medical implants. Stainless steel and nickel-titanium (NiTi) alloys exhibit different behaviors in MRI environments, impacting the clarity and accuracy of the images produced.
Artifacts Produced by Stainless Steel
Stainless steel, especially alloys with high iron content, is ferromagnetic. This ferromagnetic property causes substantial artifacts in MRI scans. These artifacts appear as distortions or voids in the images, obscuring anatomical details and hindering diagnostic accuracy, particularly in gradient-echo sequences.
Artifacts Produced by NiTi Alloys
In contrast, NiTi alloys have weak magnetic susceptibility, resulting in minimal interaction with the MRI’s magnetic fields. The minimal artifacts produced by NiTi alloys are especially useful for evaluating the patency of stented vessels and other crucial diagnostics.
Safety Considerations
Safety is a paramount concern when selecting materials for medical implants, particularly in the context of MRI compatibility. The ferromagnetic nature of stainless steel and the non-ferromagnetic properties of NiTi alloys lead to different safety profiles in MRI environments.
Safety Profile of Stainless Steel
Due to their ferromagnetic properties, stainless steel implants can pose risks during MRI procedures. These risks include:
- Movement or Displacement: The strong magnetic field of the MRI scanner can attract the stainless steel implant, potentially causing it to move or dislodge. This movement can lead to discomfort or injury.
- Heating Effects: The interaction between the stainless steel and the MRI’s radiofrequency energy can cause the implant to heat up. While generally minimal, this heating can be a concern during prolonged MRI scans.
Safety Profile of NiTi Alloys
NiTi alloys, being non-ferromagnetic, exhibit a safer profile in MRI environments. The key safety advantages include:
- Minimal Movement: NiTi implants are not attracted to the magnetic field, reducing the risk of movement or displacement during MRI procedures.
- Low Heating: NiTi alloys do not significantly absorb the radiofrequency energy, resulting in minimal heating. This makes them safer for longer MRI sessions.
Practical Applications and Case Studies
Understanding the practical applications and real-world performance of these materials in MRI settings can provide valuable insights.
Stainless Steel Implants
Stainless steel implants are common in orthopedic and cardiovascular applications because of their mechanical strength and corrosion resistance, but their use in MRI settings is limited due to significant artifacts and safety concerns.
NiTi Alloy Implants
NiTi alloy implants, such as stents and orthopedic devices, are increasingly preferred for their superior MRI compatibility. Case studies have demonstrated the successful use of NiTi stents in patients requiring regular MRI follow-ups, with minimal artifacts and no adverse safety issues.
Comparative Analysis Summary
| Feature | Stainless Steel (SS) | Nickel-Titanium (NiTi) Alloys |
|---|---|---|
| Material Composition | Primarily iron, ferromagnetic properties | Nickel and titanium, weak magnetic susceptibility |
| MRI Compatibility | Generally not MRI-compatible due to ferromagnetic nature | Compatible with MRI, minimal artifacts |
| Artifact Production | Produces significant artifacts, especially on gradient-echo imaging | Produces minor artifacts, better lumen visibility |
| Risk in MRI Environment | Risk of movement or heating due to magnetic attraction | Low risk, suitable for MRI scans without significant hazards |
| Clinical Use | Limited in MRI-compatible applications | Widely used in medical implants where MRI compatibility is needed |
Stainless steel and NiTi alloys present distinct advantages and challenges in the context of MRI compatibility. Understanding these differences is crucial for selecting the appropriate material for medical implants, ensuring both patient safety and optimal imaging outcomes.
Advancements and Emerging Materials for MRI Compatibility
Overview of Current Research Directions
Recent MRI technology advancements aim to improve the compatibility of materials for medical implants and devices. These advancements seek to reduce artifacts, enhance image quality, and guarantee patient safety during MRI procedures. Research focuses on creating materials with minimal magnetic susceptibility, low electrical conductivity, and high biocompatibility.
Promising New Materials on the Horizon
High-Impedance Flexible Coils
High-impedance flexible coils are a notable innovation in MRI technology. These coils are designed to boost the signal-to-noise ratio (SNR) and increase patient comfort. They are especially useful in wearable MR receive coils, which can be adjusted to fit different body parts. By conforming to the body’s shape, these coils offer better imaging capabilities and reduce the likelihood of artifacts.
Superparamagnetic Iron-Oxide Nanoparticles (SPIONs)
Superparamagnetic Iron-Oxide Nanoparticles (SPIONs) are being explored as alternative contrast agents for MRI. Unlike traditional gadolinium-based agents, SPIONs offer strong vessel-to-tissue contrast. They are effective at low magnetic field strengths. Their superparamagnetic properties allow for significant signal enhancement without the risks associated with gadolinium, such as nephrogenic systemic fibrosis.
Manganese-Based Contrast Agents
Manganese-based contrast agents are attracting attention due to their better safety profile compared to gadolinium. Manganese is less toxic and has been used effectively in hepatobiliary imaging, but challenges like stability issues and providing sufficient diagnostic information need to be addressed for widespread adoption. Ongoing research aims to enhance the stability and effectiveness of these agents.
Portable and Low-Field MRI Systems
The development of portable and low-field MRI systems represents a breakthrough in making MRI more accessible. These systems operate at lower magnetic field strengths, which reduces the risks associated with traditional high-field MRI. They require the development of new contrast agents and imaging strategies tailored to their unique conditions, ensuring accurate diagnostics while maintaining patient safety.
Potential Benefits and Challenges of Emerging Materials
Benefits
Firstly, improved image quality: New materials and technologies aim to minimize artifacts, resulting in clearer and more accurate images.
Secondly, enhanced patient safety: Materials with low magnetic susceptibility and minimal heating effects reduce the risk of complications during MRI procedures.
Thirdly, increased accessibility: Portable MRI systems can bring advanced imaging capabilities to remote or underserved areas, expanding the reach of medical diagnostics.
Challenges
Material stability: Ensuring the long-term stability of new materials, particularly contrast agents, is crucial for their reliable use in clinical settings.
Regulatory approval: New materials and technologies must undergo rigorous testing to meet regulatory standards for safety and efficacy.
Cost: Developing and using advanced materials and systems can be costly, which may limit their early widespread use.
Emerging materials and technologies hold great promise for improving MRI compatibility and patient outcomes. Ongoing research and development are essential to address the challenges and fully realize the potential of these innovations in clinical practice.
Frequently Asked Questions
Below are answers to some frequently asked questions:
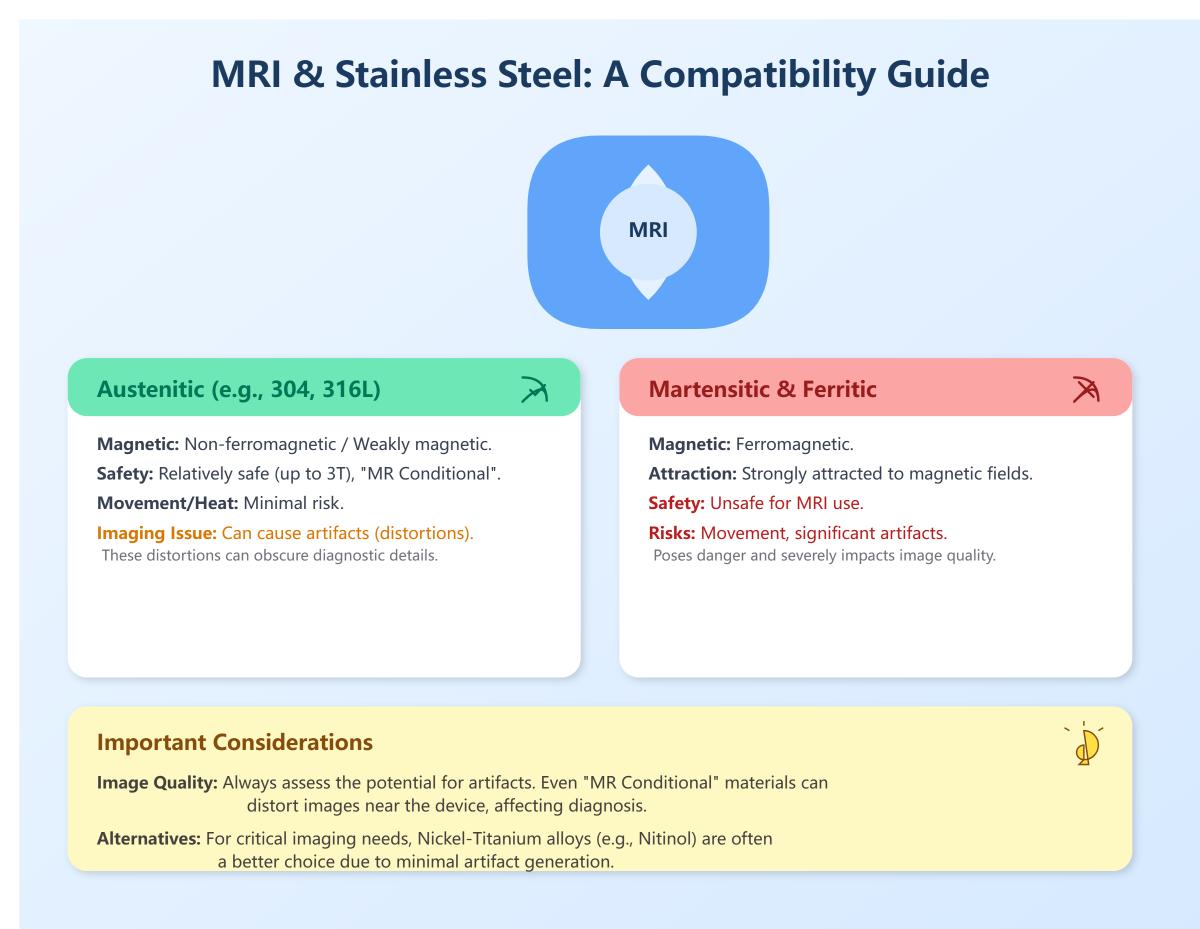
Is stainless steel compatible with MRI?
Stainless steel’s compatibility with MRI depends on its specific type and magnetic properties. Austenitic stainless steels, such as 304 and 316L, are generally considered non-ferromagnetic or weakly magnetic, making them relatively safe for use in MRI environments. These grades do not pose significant risks of movement or heating in the MRI magnetic field, especially up to 3 Tesla, and are often labeled as “MR Conditional.” However, they can cause noticeable imaging artifacts due to local magnetic field distortions, which can obscure diagnostic information.
In contrast, martensitic and ferritic stainless steels are ferromagnetic and strongly attracted to magnetic fields, making them unsafe for MRI use due to the risk of movement and significant imaging artifacts. Therefore, while certain types of stainless steel can be safely scanned under controlled MRI conditions, their impact on image quality must be carefully considered. For critical imaging near stainless steel devices, alternative materials like nickel-titanium alloys, which exhibit minimal artifacting, may be preferred.
What are the differences between stainless steel and NiTi in MRI compatibility?
Stainless steel and nickel-titanium (NiTi) alloys exhibit different behaviors in MRI environments due to their distinct material properties. Stainless steel, particularly the 316L variant, contains iron and chromium, making it ferromagnetic. This ferromagnetic nature leads to significant artifacts in MRI scans, especially with gradient-echo imaging, causing signal loss and reduced visibility of surrounding structures. Consequently, stainless steel can obscure diagnostic details, limiting its utility in MRI settings.
In contrast, NiTi alloys, known for their shape memory and superelastic properties, have lower magnetic susceptibility. This results in minimal artifact production, maintaining clearer visibility of tissues and implants in MRI scans. NiTi’s superior MRI compatibility makes it more suitable for applications where precise imaging is crucial post-implantation. Additionally, NiTi alloys pose fewer safety concerns compared to stainless steel, as they interact weakly with magnetic fields, making them generally safer for patients undergoing MRI.
How do MRI artifacts affect diagnostic imaging?
MRI artifacts significantly impact diagnostic imaging by distorting or obscuring anatomical and pathological features. Metallic implants like stainless steel cause various artifacts such as signal loss, geometric distortion, failure of fat suppression, bright pile – up artifacts, and can obscure pathology. These artifacts reduce image clarity, complicate the diagnosis of conditions near the implant, and pose challenges for follow – up scans. However, strategies like pulse sequence optimization, short echo times, and advanced techniques can mitigate their impact.
What materials are best for MRI-compatible medical implants?
When considering materials for MRI-compatible medical implants, it is essential to focus on non-ferromagnetic and non-conductive materials to avoid risks such as displacement or heating due to the magnetic field and radiofrequency (RF) energy. Titanium and its alloys are highly favored for MRI compatibility because of their non-ferromagnetic properties, high strength, and biocompatibility. Titanium alloys like Ti-6Al-4V are particularly preferred due to their enhanced mechanical properties while maintaining MRI compatibility.
Polymers and ceramics are inherently MRI safe as they do not contain metals and therefore do not interact with the MRI’s magnetic field, making them suitable for various medical implants. Some stainless steel alloys, such as 316L, are considered MR Conditional, meaning they can be used under specific conditions but may produce artifacts and pose heating risks.
Are there any new materials being developed for MRI compatibility?
Yes, new materials are being developed for MRI compatibility. Researchers at Penn State are working on a novel ceramic material to enhance MRI signals, enabling shorter scan times and higher resolution. For implantable devices, polymers and certain ceramics are favored due to their low electrical conductivity, reducing RF – induced heating. The development also involves adhering to strict testing standards and exploring alternative testing methods, such as computational models, to ensure patient safety and improve MRI – compatible technology.
How do regulations ensure the safety of MRI-compatible materials?
Regulations ensure the safety of MRI-compatible materials by setting strict guidelines and standards for their evaluation and use. The U.S. Food and Drug Administration (FDA) categorizes devices based on their safety in MRI environments as MR Safe, MR Conditional, or MR Unsafe. MR Safe devices pose no known hazards, MR Conditional devices are safe under specified conditions, and MR Unsafe devices should be avoided due to significant risks.
To determine these classifications, materials undergo rigorous testing to assess their interactions with MRI’s strong magnetic fields and radiofrequency energy. Organizations like the American Society for Testing and Materials (ASTM) provide standardized tests for magnetically induced displacement and heating effects.
Additionally, MRI facilities enforce safety zones to control access and ensure only compatible materials enter the MRI environment. Comprehensive screening processes, including medical history reviews and metal detection, help identify potential risks. These regulations and practices collectively minimize hazards, ensuring patient safety during MRI procedures.
