How can you be sure the copper you’re working with is the real deal? Whether it’s for crafting electrical components or building durable structures, the purity of copper directly impacts performance, safety, and longevity. Yet, distinguishing pure copper from alloys or contaminated materials isn’t always straightforward. From simple visual cues to advanced chemical analysis, there are a variety of methods to verify copper’s quality—each with its own level of accuracy, complexity, and cost. But which approach is right for your needs? And how can you strike the perfect balance between precision and practicality? Let’s explore the most effective techniques for testing copper purity and uncover the tools and tips that will ensure your materials meet the highest standards.
Visual Inspection for Testing Copper Purity
Characteristics to Look for in Pure Copper
Color and Appearance
Pure copper is easily recognized by its reddish-brown or orange hue, which develops a greenish-blue patina over time when exposed to air. This distinctive patina is a natural result of oxidation and serves as a hallmark of copper’s authenticity.
Surface Uniformity and Reflectivity
High-purity copper typically exhibits a smooth, reflective surface with consistent coloration throughout. When scratched or cut, the material retains its uniform reddish tone, indicating minimal contamination. Irregular textures, dull finishes, or color variations may signal impurities or the presence of alloys, which can compromise the copper’s quality.
Detecting Impurities Through Visual Cues
Signs of Impurities
Impurities in copper often appear as discoloration, streaks, or uneven patches on the surface. Such imperfections may arise from manufacturing defects or contamination, suggesting the need for further testing. Additionally, if the copper does not develop a patina over time, it could indicate alloying or surface coatings that inhibit oxidation, pointing to lower-quality material.
Tools and Techniques for Enhanced Visual Analysis
Use of Magnification
Using tools like magnifying glasses or microscopes can help identify subtle imperfections or irregularities on the copper surface. These devices offer a closer view, making it easier to detect small flaws or inconsistencies.
Lighting Conditions
Good lighting is essential for accurately assessing copper’s appearance. Bright, natural light or well-controlled artificial lighting ensures the material’s true color and surface details are visible without distortion.
Surface Preparation
Before inspection, cleaning the copper surface can remove dirt, grease, or oxidation, allowing for a clearer evaluation of its properties. Use mild solvents or abrasives to clean the material carefully, avoiding any alterations to its natural appearance.
Physical and Conductivity Testing: Evaluating Copper’s Properties
Physical Property Testing
Density Testing
Density is a fundamental physical property used to determine copper purity. Pure copper has a density of approximately 8.96 g/cm³. This test measures the sample’s mass and volume, often determined using the water displacement method. By submerging the copper sample in water and measuring the displaced volume, an accurate density calculation can be obtained. Significant deviations from the standard density value may indicate impurities or alloying elements.
Magnetism Test
Pure copper is non-magnetic, making magnetism testing a quick and straightforward way to identify contamination. If the sample exhibits magnetic properties, it likely contains ferromagnetic impurities such as iron or nickel. This test requires only a magnet for verification.
Hardness and Strength Testing
Tests like Brinell or Vickers measure how resistant copper is to deformation, providing insight into its mechanical properties. Pure copper displays moderate hardness and excellent ductility, while alloys often exhibit higher hardness but reduced malleability. Tensile strength testing, which involves stretching the material until it breaks, further highlights the differences; pure copper typically shows high elongation and lower fracture resistance compared to alloys.
Conductivity Testing
Electrical Conductivity Measurement
Specialized meters are used to measure how well copper conducts electricity. Pure copper has a conductivity of approximately 100% IACS (International Annealed Copper Standard). Impurities can reduce this conductivity, making the test an essential method for verifying copper’s suitability for electrical applications.
Resistance and Temperature Testing
Resistance tests using a multimeter provide practical evaluations of copper’s conductivity, with pure copper having a resistance of approximately 1.68 × 10⁻⁸ ohm-meters at room temperature. However, conductivity can vary with temperature, so tests should be done in controlled conditions to avoid temperature-related inaccuracies.
Eddy Current-Based Testing
This method is ideal for thin copper sheets or wires, providing precise, non-destructive measurements. High-frequency alternating currents are used to evaluate conductivity, making it an efficient and reliable option for assessing copper purity without damaging the material. Tools like the SIGMATEST system are commonly employed for this purpose, ensuring accurate results in industrial applications.
Chemical Testing: Ensuring Copper Purity with Precision
Overview of Chemical Analysis Methods
Chemical testing methods provide a detailed assessment of copper purity by identifying and quantifying impurities at a molecular level. These methods are vital for precision applications where small impurities can significantly affect performance. Industries such as electronics, construction, and manufacturing rely on these techniques to meet stringent quality standards.
Spectrometry for Accurate Results
Atomic Emission and Absorption Spectrometry
Spectrometry is a common and effective technique for assessing copper purity. It measures the interaction of light with the atoms in a sample, offering exceptional precision for detecting trace impurities.
- Procedure: The sample is vaporized, and its atoms are exposed to a light source or plasma. Specific wavelengths of light are analyzed to detect elements in the sample.
- Applications: This method is ideal for identifying impurities like lead, arsenic, or iron, even at low concentrations.
- Advantages: High sensitivity and rapid analysis.
- Limitations: Requires expensive equipment and skilled operators.
X-Ray Fluorescence (XRF)
XRF spectrometry uses high-energy X-rays to excite atoms in the copper sample, causing them to emit secondary X-rays. These emissions are then analyzed to determine the sample’s composition.
- Procedure: A sample is exposed to X-rays, and the emitted fluorescence is measured to identify and quantify elements.
- Advantages: Non-destructive and capable of analyzing solid samples directly.
- Limitations: Less effective for detecting elements in very low concentrations compared to AES or AAS.
Electrolytic Methods for Precision
Electrolytic testing is a highly accurate approach for determining copper purity. This method involves dissolving a copper sample in an acid solution and using electrochemical processes to isolate and measure its copper content.
- Procedure: A sample is dissolved in a mixture of sulfuric and nitric acids. Copper ions are then plated onto a platinum cathode through electrodeposition, and the weight of the deposited copper is measured to calculate purity.
- Applications: Commonly used in laboratories to test copper purity levels ranging from 99.75% to 99.95%.
- Standards: Often performed in accordance with ASTM E53 for reproducible results.
- Advantages: Exceptional accuracy for high-purity copper.
- Limitations: Requires specialized equipment and significant preparation time.
Chromatography for Impurity Identification
Chromatography is used to separate and analyze the chemical components of a copper sample. This method is particularly effective for identifying organic impurities or trace elements.
- Procedure: The copper sample is dissolved in a solvent and passed through a chromatography column. Different components separate based on their interactions with the stationary and mobile phases.
- Advantages: Offers detailed analysis of complex mixtures and impurities.
- Limitations: Requires advanced laboratory equipment and expertise, making it unsuitable for on-site testing.
Factors Influencing Chemical Testing Accuracy
Proper sample preparation in a clean, controlled environment is essential to avoid contamination and ensure reliable results. Regular calibration of instruments like spectrometers and chromatographs is also critical for maintaining accuracy and consistency. Skilled technicians are necessary to interpret data and address potential errors in the process. Environmental factors like temperature and humidity can impact the accuracy of sensitive methods such as spectrometry, making controlled conditions vital for precise outcomes.
Advantages of Chemical Testing for Industrial Applications
Chemical testing methods are indispensable for industries where copper purity directly impacts performance and reliability. Their ability to detect even minute impurities ensures that copper meets the stringent requirements of applications such as electrical wiring, heat exchangers, and semiconductors. These methods also play a key role in quality control, helping manufacturers comply with international standards and certifications.
Laboratory vs. DIY Testing: Choosing the Right Approach
Laboratory Testing
Laboratories use advanced methods like spectrometry (AES, AAS, and XRF), electron microscopy, and metallographic analysis to identify trace impurities. These techniques provide detailed insights into the chemical composition and physical properties of copper, ensuring precise evaluations for critical applications.
Key Advantages
- High Accuracy: Laboratory tests can detect impurities down to parts per million, making them essential for industries like electronics and aerospace, where precision is paramount.
- Comprehensive Analysis: Methods such as XRF and atomic absorption spectrometry reveal a full breakdown of the material’s composition, including minor and trace elements.
- Standards Compliance: Accredited laboratories follow recognized standards like ISO 17025, ensuring results are consistent, reliable, and traceable.
- Advanced Capabilities: Specialized techniques, such as electron microscopy and electrolytic analysis, provide insights beyond the scope of basic testing methods.
Limitations to Consider
- Cost: Professional testing can be expensive, especially for small-scale operations or individuals with limited budgets. Costs often include sample preparation and shipping.
- Time: Laboratory testing may take longer due to the complexity of procedures and the need for high precision.
- Accessibility: People in remote areas or smaller businesses may face challenges accessing accredited laboratories, especially when quick results are needed.
DIY Testing
DIY testing methods offer a practical alternative for those prioritizing cost-effectiveness and convenience. These methods are especially helpful for quick checks or when extreme precision isn’t required.
Common DIY Techniques
- Visual Inspection: Observing the color, texture, and patina of copper can provide immediate clues about its purity. Using magnifying tools can improve accuracy.
- Conductivity Testing: Measuring electrical conductivity with a multimeter or eddy current device helps assess purity, as pure copper typically has 100% IACS conductivity.
- Density Testing: The water displacement method calculates density, with pure copper expected to have a density of 8.96 g/cm³.
- Magnetism Test: Copper is non-magnetic, so the presence of magnetic properties may indicate contamination by ferromagnetic materials.
Benefits of DIY Testing
- Cost-Effective: DIY methods are affordable, requiring minimal investment in tools or equipment.
- Fast Results: Most tests can be performed in minutes, offering immediate insights without delays.
- Convenience: Testing can be done on-site, eliminating the need to send samples to external facilities.
Drawbacks of DIY Testing
- Limited Accuracy: DIY methods cannot match the precision of laboratory testing and may miss trace impurities or complex alloy compositions.
- Skill Dependency: Results depend on the user’s skill, environmental factors, and equipment accuracy.
- Restricted Scope: DIY techniques lack the ability to provide detailed chemical breakdowns or advanced material characterizations.
Factors to Consider When Choosing Between Laboratory and DIY Testing
The decision between laboratory and DIY testing depends on factors like the application, budget, and time sensitivity. High-stakes industries, such as aerospace, require laboratory-grade precision, while less critical applications may benefit from the speed and affordability of DIY methods.
Portable eddy current devices are improving DIY accuracy while staying convenient, making them a valuable option for individuals seeking a balance between cost and reliability.
Importance of Copper Purity in Industrial Applications
Impact on Electrical and Thermal Conductivity
Applications that demand excellent electrical and thermal conductivity rely on high-purity copper. Pure copper reduces energy losses in power transmission, making systems more efficient. Its exceptional thermal conductivity also makes it indispensable in heat exchangers, radiators, and industrial cooling systems. Even trace impurities can significantly compromise performance, leading to increased costs and reduced efficiency.
Role in Plumbing and Fluid Systems
Copper’s antimicrobial and corrosion-resistant properties make it ideal for plumbing, ensuring clean water delivery and preventing bacterial growth. This resistance to chemical deterioration protects pipelines and fittings, maintaining their integrity under varying pressure and temperature conditions. As a result, high-purity copper remains a reliable choice for fluid systems in both residential and industrial applications.
Applications in Manufacturing and Construction
In manufacturing, high-purity copper is critical for processes such as welding, machining, and forming, where impurities can weaken welds and reduce ductility. Its mechanical performance ensures components meet stringent industry demands. In construction, copper is valued for its durability and ability to resist environmental factors, making it a dependable material for roofing, cladding, and electrical wiring. Using pure copper ensures safety, reliability, and adherence to regulatory standards.
Importance in Renewable Energy Systems
Renewable energy systems like solar panels and wind turbines depend on copper for efficient power generation and transmission. High-purity copper plays a vital role in wiring, connectors, and thermal management systems, optimizing the performance of these technologies. Ensuring copper purity is essential for the reliability and effectiveness of sustainable energy solutions.
Environmental and Sustainability Benefits
The sustainability of copper is closely tied to its purity. Pure copper is easier to recycle, reducing waste and minimizing environmental impact. Its ability to be reclaimed with minimal degradation supports eco-friendly manufacturing practices and a circular economy. Furthermore, the superior conductivity of high-purity copper contributes to energy-efficient systems, reducing the carbon footprint across industries.
Meeting Industry Standards and Certifications
Compliance with stringent international standards ensures that copper maintains its purity and performance in demanding applications. This ensures high-purity copper supports reliable components for critical sectors such as aerospace, automotive, and telecommunications. By adhering to these benchmarks, manufacturers can deliver products that meet safety, quality, and durability requirements.
Frequently Asked Questions
Below are answers to some frequently asked questions:
What are the most effective methods to test copper purity?
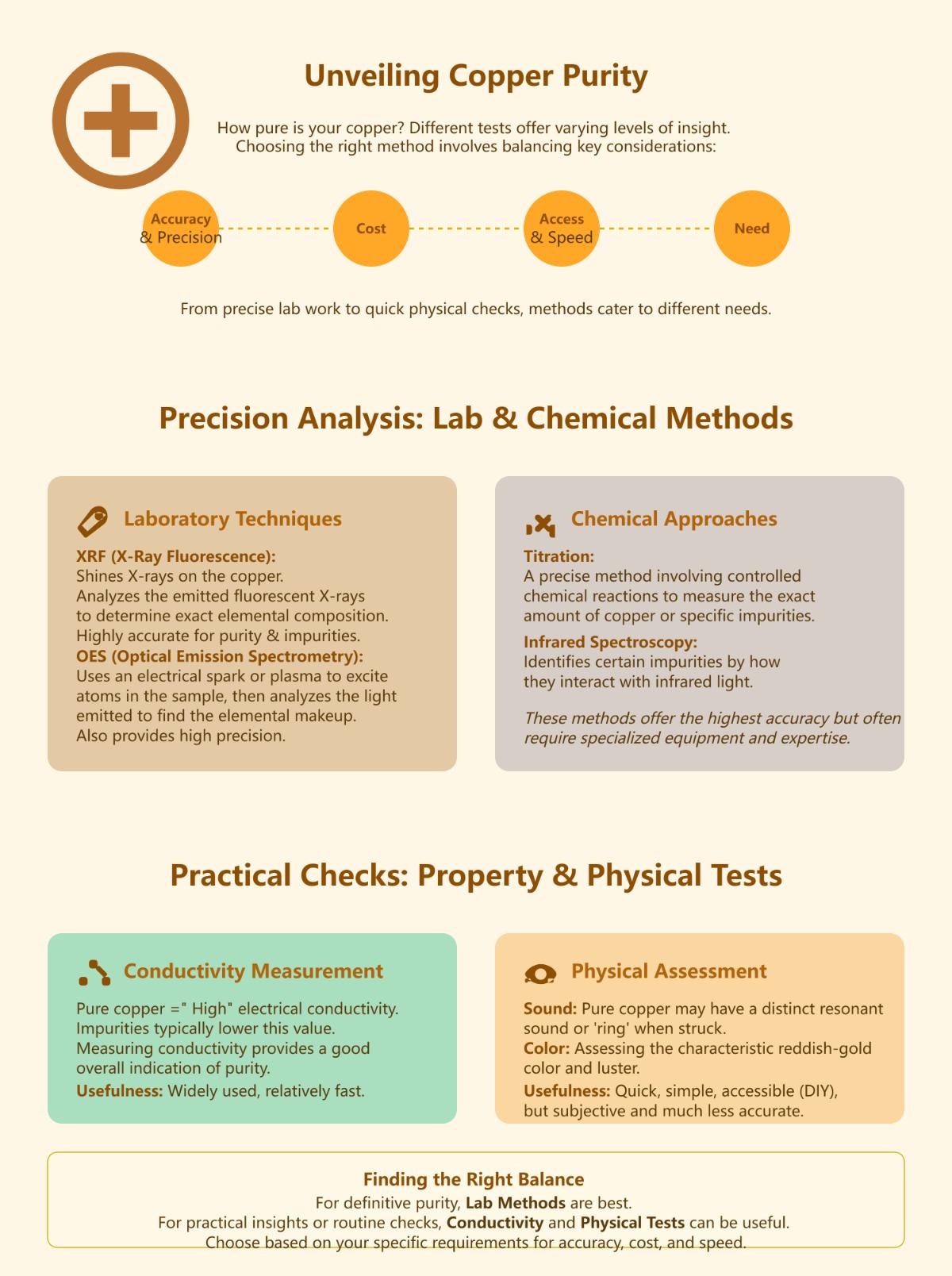
The most effective methods to test copper purity include laboratory techniques like X-Ray Fluorescence (XRF) and Optical Emission Spectrometry (OES), which provide accurate analysis of elemental composition and impurities. Conductivity measurements are widely used, as pure copper exhibits high electrical conductivity. Chemical methods like titration and infrared spectroscopy offer precise impurity detection, while physical tests such as sound and color analysis provide quick, though less accurate, assessments. For comprehensive results, laboratory methods are preferred, but DIY approaches can offer practical insights, as discussed earlier. Each method is suited to specific needs, balancing cost, precision, and accessibility.
How does conductivity testing confirm copper quality?
Conductivity testing confirms copper quality by measuring its ability to conduct electricity, which directly correlates with purity. Pure copper exhibits high electrical conductivity, and deviations often indicate impurities. This method uses conductivity meters to calculate resistance and conductivity, applying Ohm’s Law. Factors like temperature and surface conditions are critical for accurate results, as oxidation or contamination can interfere with measurements. Conductivity testing is efficient, precise, and non-destructive, making it superior to visual or density tests. For comprehensive analysis, it can be combined with other methods, ensuring reliable assessment of copper purity for industrial applications.
Can chemical testing detect all impurities in copper?
Chemical testing is highly effective for detecting impurities in copper, offering precise and detailed analysis through methods like atomic absorption spectroscopy (AAS) and inductively coupled plasma mass spectrometry (ICP-MS). However, it may not detect all impurities, especially trace organic contaminants or those requiring specialized techniques like chromatography. The effectiveness depends on the testing method and the required purity level. Combining multiple approaches, such as chemical, spectroscopic, and physical analyses, enhances accuracy and ensures comprehensive impurity detection, making it essential to tailor the method to the specific application and purity standards.
What are the differences between laboratory and home testing methods for copper purity?
Laboratory testing methods for copper purity, such as XRF, ICP-OES, and AAS, provide highly accurate and detailed analyses, detecting trace impurities and requiring specialized equipment and skilled operators. In contrast, home testing methods, including visual inspection, density tests, magnet tests, and conductivity measurements, are simpler and rely on basic tools but lack precision and cannot identify microscopic impurities. While laboratory tests ensure reliable and repeatable results, home methods are suitable for quick, preliminary assessments. The choice between these approaches depends on the required accuracy, budget, and application, as discussed earlier.
Why is copper purity critical for industrial applications?
Copper purity is vital for industrial applications because it directly affects performance, efficiency, and reliability. Pure copper ensures optimal electrical conductivity, critical for wiring and electronics, while impurities can reduce efficiency and durability in construction and machinery. It also retains antimicrobial properties, essential for plumbing and medical equipment. Impurities may compromise these benefits and lead to premature failure or reduced functionality. As discussed earlier, rigorous testing methods, such as XRF, OES, and ICP-MS, are used to verify purity, ensuring compliance with industry standards and safeguarding the quality of copper-based products across various sectors.
Are there any emerging trends in copper testing and quality control?
Emerging trends in copper testing and quality control include the adoption of advanced corrosion testing methods like Conductive Deposit Test (CDT) and Wire Corrosion Test (WCT) for detailed material analysis, the use of automated and quantitative techniques to improve accuracy and reproducibility, and increased standardization through international guidelines such as ASTM and ISO. Additionally, technological innovations such as AI-driven mining processes and sustainable practices like hydrometallurgical processing and copper recycling are transforming the industry. These advancements emphasize precision, efficiency, and environmental responsibility, addressing the growing demand for high-quality copper in various applications.
