
Non-ferrous metals refer to all metals other than iron and iron-based alloys. They possess many excellent characteristics and play an extremely important role in industrial fields, especially in high-tech areas.
I. Aluminum and Aluminum Alloys
1. Aluminum (Al)
Pure aluminum can be classified into high-purity aluminum, industrial high-purity aluminum, and industrial pure aluminum based on aluminum content. High-purity aluminum has an aluminum mass fraction of 99.3% to 99.996% and is mainly used in scientific experiments, chemical industry, and other fields.
Industrial high-purity aluminum has an aluminum mass fraction of 99.85% to 99.9% and is mainly used for preparing aluminum-based alloys. Pure aluminum can be used to make electrical wires, aluminum boxes, shielding casings, and chemical containers, etc.
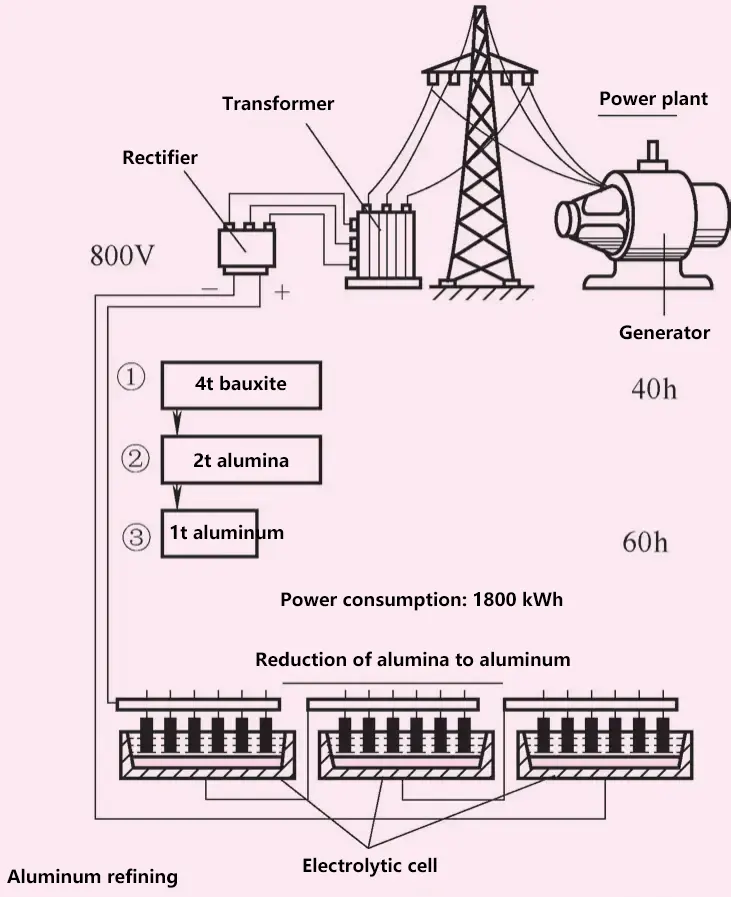
(1) Ore deposits and smelting
Pure metallic aluminum does not exist in nature; aluminum exists in compound form and is the metal with the largest reserves (about 8% of the Earth’s crust). Bauxite is the mineral with the highest aluminum content; corundum is crystalline alumina; gems (ruby, sapphire, yellow sapphire, purple sapphire) are pure, transparent alumina.
(2) Main properties
- Physical properties. Melting point is 658°C, density is 2.7kg/cm 3 , and electrical conductivity is second only to silver and copper.
- Chemical properties. Corrosion-resistant, with a thick oxide film layer.
- Mechanical properties. Tensile strength for cast aluminum is 90-120MPa, for rolled aluminum is 150-230MPa. Elongation is 20% to 35%.
- Technological properties. Aluminum can be forged, rolled, drawn, machined, cast, welded, and riveted.
2. Aluminum alloys
Aluminum alloys mainly add copper, silicon, magnesium, manganese, and zinc as alloying elements.
(1) Cast aluminum alloys
They have very good casting properties, can maintain their stability under climate and seawater effects, and can be machined and welded.
(2) Wrought aluminum alloys
They have good mechanical properties and are suitable for deformation processing. Semi-finished products available in the market include aluminum plates, strips, tubes, bars, extruded aluminum parts, and die forgings.
Aluminum alloys are used in the construction industry for making doors, windows, and structural components; in the food industry, storage tanks, cans, beverage containers, and most everyday pots and pans are made of aluminum.
3. Grade designations
1) The grade designation method for cast aluminum and aluminum alloys is as follows.
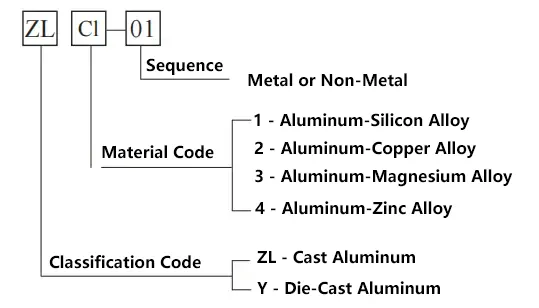
Cast aluminum alloys include ZL102, ZL105, ZL201, ZL401, etc.
2) Wrought aluminum and aluminum alloy grades are represented by four-digit codes, where the first, third, and fourth digits are numbers, and the second is a letter. The first digit indicates the group of aluminum and aluminum alloy,
as shown in the table below. The second letter indicates the modification status of the original pure aluminum or aluminum alloy, and the last two digits are used to identify different aluminum alloys within the same group or indicate the purity of aluminum.
Groups of aluminum and aluminum alloys:
| Group | Grade series |
| Pure aluminum (aluminum mass fraction ≥99.0%) | 1××× |
| Aluminum alloys with copper as the main alloying element | 2××× |
| Aluminum alloys with manganese as the main alloying element | 3××× |
| Aluminum alloys with silicon as the main alloying element | 4××× |
| Aluminum alloys with magnesium as the main alloying element | 5××× |
| Aluminum alloys with magnesium and silicon as the main alloying elements and Mg 2 Si phase as the strengthening phase | 6××× |
| Aluminum alloys with zinc as the main alloying element | 7××× |
| Aluminum alloys with other elements as the main alloying elements | 8××× |
| Reserved alloy group | 9××× |
3) The comparison between new and old grade designations for wrought aluminum and aluminum alloys is shown in the table below.
Comparison of new and old grade designations for wrought aluminum and aluminum alloys:
| Category | Old grade | New grade |
| Corrosion-resistant aluminum alloy | LF2 | 5A02 |
| LF21 | 3A21 | |
| Hard aluminum alloy | LY11 | 2A11 |
| LY12 | 2A12 | |
| LY8 | 2B11 | |
| Extra-hard aluminum alloy | LC3 | 7A03 |
| LC4 | 7A04 | |
| LC9 | 7A09 | |
| Forged aluminum alloy | LD5 | 2A50 |
| LD7 | 2A70 | |
| LD8 | 2A80 | |
| LD10 | 2A14 |
4) The comparison between new and old grade designations for industrial pure aluminum is shown in the table below.
Comparison of new and old grade designations for industrial pure aluminum:
| Old grade | L1 | L2 | L3 | L4 | L5 |
| New grade | 1070 | 1060 | 1050 | 1035 | 1200 |
4. Processing properties
Aluminum alloys can undergo machining or non-cutting processing. Cutting speeds can reach up to 400m/min, saving processing time. High-speed steel and carbide tools are used for machining.
Cutting fluids and lubricants used include oil, turpentine, alcohol, and soapy water. Hot deformation processing must comply with strict temperature specifications. Aluminum has high thermal conductivity and thermal expansion rate, and welding doesn’t present any special difficulties. Anodizing, acid pickling, and coating can improve corrosion resistance.
II. Copper and Copper Alloys
1. Copper (Cu)
Apart from aluminum, copper is the most important non-ferrous metal. Industrial pure copper is rose-red in color, turning purple-red when an oxide film forms on the surface. Copper is an indispensable metal for electrical engineering and mechanical manufacturing.
(1) Ore deposits and smelting
Copper primarily exists in ore form, with the main copper ores being chalcocite (Cu 2 S) and chalcopyrite (CuFeS 2 ). Sulfur is removed in roasting furnaces, and pure copper is obtained through furnace refining or electrolysis.
(2) Main properties
1) Physical properties.
Melting point is 1084°C, density is 8.9kg/cm 3 , thermal conductivity is 8 times higher than steel, and electrical conductivity is 7 times higher than steel.
2) Chemical properties.
Due to the thick oxide film layer, it has high resistance to air and water corrosion. It reacts with carbon dioxide in the air to form copper carbonate (green patina).
Tensile strength ≤250MPa, average elongation of copper wire is 30% to 50%, hardness is only about 25% of steel.
4) Technological properties.
Copper can be forged, rolled, spun, drawn, machined, cast, welded, etc.
2. Copper alloys
Copper alloys include binary alloys and multi-element alloys, with alloying elements such as zinc, tin, nickel, aluminum, and iron.
(1) Brass (copper-zinc alloy)
It has good casting properties, machinability, corrosion resistance, and cold formability. Strength increases with zinc content. Brass with other alloying elements added is called special brass. Common alloying elements include aluminum, iron, silicon, manganese, lead, tin, nickel, etc., which can improve certain properties of brass.
(2) Bronze
It has a grayish-green color, hence the name bronze. To improve the alloy’s technological and mechanical properties, most bronzes also contain other alloying elements such as lead, zinc, phosphorus, etc.
Since tin is a scarce element, industry also uses many tin-free bronzes. The main tin-free bronzes include aluminum bronze, beryllium bronze, manganese bronze, silicon bronze, etc.
Tin bronze has good mechanical properties, corrosion resistance, friction reduction, and casting properties; tin bronze has better corrosion resistance than brass in atmosphere, seawater, fresh water, and steam.
Aluminum bronze has better mechanical properties, wear resistance, corrosion resistance, cold resistance, heat resistance than tin bronze, is non-magnetic, has good fluidity, no tendency to segregate, and can produce dense castings. Adding iron, nickel, and manganese to aluminum bronze can further improve various properties of the alloy.
(3) Cupronickel
Copper-based alloys with nickel as the main additive element appear silver-white, hence called cupronickel. Copper-nickel binary alloys are called ordinary cupronickel, while copper-nickel alloys with manganese, iron, zinc, and aluminum are called complex cupronickel. Adding nickel to pure copper significantly improves strength, corrosion resistance, electrical resistance, and thermoelectric properties.
Industrial cupronickel is divided into structural cupronickel and electrical cupronickel based on performance characteristics and uses, meeting various corrosion resistance and special electrical and thermal properties requirements.
3. Grade designations
(1) Pure copper grade designation method
Pure copper grade designation method:
| Grade | Designation | Code | Chemical composition mass fraction (%) | |||
| Cu (not less than) | Impurities | Total impurities | ||||
| Bi | Pb | |||||
| Pure copper | No. 1 copper | T1 | 99.95 | 0.001 | 0.003 | 0.05 |
| No. 2 copper | T2 | 99.90 | 0.001 | 0.005 | 0.1 | |
| No. 3 copper | T3 | 99.70 | 0.002 | 0.01 | 0.3 | |
| Oxygen-free copper | No. 1 oxygen-free copper | TU1 | 99.97 | 0.001 | 0.003 | 0.03 |
| No. 2 oxygen-free copper | TU2 | 99.95 | 0.001 | 0.004 | 0.05 | |
(2) Copper alloys
1) Brass.
Ordinary brass: Designation uses “H + copper percentage content”, where “H” stands for ordinary brass.
Ordinary brass:
| Code | Cu mass fraction (%, not less than) | Impurities mass fraction (%) |
| H96 | 95.0~97.0 | ≤0.2 |
| H90 | 88.0~91.0 | ≤0.2 |
| H80 | 79.0~81.0 | ≤0.3 |
| H68 | 67.0~70.0 | ≤0.3 |
Special brass: Designation uses “H + main additive element symbol + copper content percentage + main additive element content percentage”.
Cast copper alloys: Designation is “ZCu + main additive element symbol + main additive element percentage content + other element symbols and percentage contents”, where “Z” stands for casting, such as ZCuSn10Zn2, ZCuPb10, ZCuZn40Mn2, ZCuZn33Pb2, etc.
Special brass:
| Special brass | Code | Main chemical composition mass fraction (%) | ||
| Cu (not less than) | Other impurity elements | Total impurities | ||
| Leaded brass | HPb63-3 HPb59-1 | 62.0~65.0 57.0~60.0 | Lead 2.4~3.0 Lead 0.8~1.9 | ≤0.75 ≤1.0 |
| Tin brass | HSn62-1 | 61.0~63.0 | Tin 0.7~1.1 | ≤0.3 |
| Arsenic-added brass | HSn70-1 | 69.0~71.0 | Tin 0.8~1.3, Arsenic 0.03~0.06 | ≤0.3 |
| Aluminum brass | HAl60-1-1 | 58.0~61.0 | Aluminum 0.7-1.5, Arsenic 0.1-0, Iron 0.7-1.5 | ≤0.7 |
| Iron brass | HFe59-1-1 HFe58-1-1 | 57.0~60.0 56.0~58.0 | Iron 0.6~1.2, Aluminum 0.1~0.5 Manganese 0.5~0.8, Tin 0.3~0.7 | ≤0.3 ≤0.5 |
| Manganese brass | HMn58-2 | 57.0~60.0 | Manganese 1.0~2.0 | ≤1.2 |
| Nickel brass | HNi65-5 | 64.0~67.0 | Nickel 5.0~6.5 | ≤0.3 |
| Silicon brass | HSi80-3 | 79.0~81.0 | Silicon 2.5~4.0 | ≤1.5 |
2) Bronze.
Designation uses “Q + main additive element symbol and percentage content + other element symbols and percentage contents”.
Bronze:
| Name | Designation | ||
| Bronze | Tin bronze | QSn4-3, QSn4-4-2.5, QSn6.5-0.1, QSn6.5-0.4 | |
| Tin-free bronze | Aluminum bronze | QAl5, QA17, QA19-2, QA19-4, QAl10-3-1.5 | |
| Manganese bronze | QMn1.5, QMn5 | ||
| Silicon bronze | QSi1-3, QSi3-1 | ||
| Beryllium bronze | QBe2 | ||
3) Cupronickel.
Cupronickel designation uses “B + nickel percentage content”, such as B5, where nickel mass fraction is around 5%.
Special cupronickel designation uses “B + main additive element symbol + nickel percentage content”, such as BFe11-1-1 for iron cupronickel with nickel mass fraction around 11%; BMn40-1.5 for manganese cupronickel with nickel mass fraction around 40%; BAl13-3 for aluminum cupronickel with nickel mass fraction around 13%.
4. Processing properties
Through cold working deformation, strength and hardness increase significantly, while elongation decreases accordingly; after softening annealing, elongation increases, while strength and hardness decrease.
III. Zinc and Zinc Alloys
1. Zinc (Zn)
When zinc is alloyed with copper, it can produce gold-like alloys. Zinc has
(1) Ore deposits and smelting
Zinc deposits include sphalerite (ZnS), smithsonite (ZnCO₃), and hemimorphite [Zn₄Si₂O₇(OH)₂·H₂O]. Commercial zinc on the market contains 99.5% zinc by mass, and high-purity zinc (99.997% by mass) can be obtained through distillation and electrolysis.
(2) Main properties
1) Physical properties.
The melting point is 419.5°C, boiling point is 911°C, density is 7.14kg/cm³, and Mohs hardness is 2.5.
2) Chemical properties.
It has good corrosion resistance and forms a thick layer of zinc oxide (ZnO) when combined with oxygen.
3) Mechanical properties.
Grain strength ≤140MPa. Zinc is very brittle, easily workable at 120°C, and becomes brittle again when the temperature rises to 205°C. During galvanization, zinc bonds well with the base metal.
4) Processing properties.
Used as a surface protection material (hot-dip galvanizing, spray galvanizing, or electroplating), zinc can be used as an excellent alloying element. When processing zinc, it is advisable to use a single-cut file. Zinc has good forging properties. Commercial zinc products include zinc ingots, bars, zinc sheets, and wire materials.
2. Zinc alloys
Zinc alloys are alloys composed of zinc as the base with the addition of other elements. Common alloying elements include aluminum, copper, magnesium, cadmium, lead, and titanium.
(1) Cast zinc alloys
Formed by casting, they have good casting properties and geometric accuracy retention. Suitable for die-casting instruments, automotive parts, and casings.
(2) Wrought zinc alloys
Zinc alloys used to produce various shapes of zinc materials. Often include small amounts of cadmium, lead, iron, titanium, and copper. Mainly used for battery casings, printed circuit boards, roofing sheets, and daily hardware items.
3. Grade designations
1) Zinc ingot grades are expressed as “Zn + zinc percentage content”, such as Zn99.95.
2) Cast zinc alloy grades are expressed as “ZZn + other element symbols and percentage content”, such as ZZnAl6Cu1, ZZnAl4Cu1Mn.
3) Die-cast zinc alloy grades are expressed as “YZZn + other element symbols and percentage content”, such as YZZnAl4Cu1.
4) Other zinc alloy grade designations: Battery zinc plate grades are expressed as “XDx”, where “x” is a number indicating the sequence, such as XD1; Offset zinc plate grades are expressed as “XJx”, where “x” is a number indicating the sequence, such as XJ1; Zinc cake grades are expressed as “XBx”, where “x” is a number indicating the sequence, such as XB1.
4. Processing properties
Through cold working deformation, strength and hardness increase significantly, while elongation decreases accordingly. After softening annealing, elongation increases, while strength and hardness decrease.
IV. Magnesium and Magnesium Alloys
1. Magnesium (Mg)
(1) Deposits and smelting
Magnesium holds an important position among chemical elements. By processing ores (magnesite, dolomite, carnallite), CO₂ is removed from magnesite (MgCO₃) to obtain magnesium oxide (MgO). Magnesium is obtained through electrolysis.
(2) Main properties
1) Physical properties.
The melting point is 657°C, and the density is 1.74kg/cm³.
2) Chemical properties.
Very stable in dry air. In pyrotechnics, magnesium is combined with oxygen to produce a flash; burning magnesium can only be extinguished with sand, as water would intensify the oxidation reaction.
3) Mechanical properties.
Pure magnesium has a very low tensile strength of 110-200MPa.
4) Processing properties.
Easy to machine, allowing for relatively high cutting speeds, with good formability and casting properties.
2. Magnesium alloys
Due to the flammability and low strength of pure magnesium, only magnesium alloys are used in engineering. Magnesium alloys are the lightest metal structural materials. The following alloying elements have a significant impact on the properties of magnesium alloys.
- Manganese: Improves corrosion resistance.
- Aluminum: Enhances mechanical properties.
- Zinc: Increases ductility and strength.
(1) Cast magnesium alloys
Magnesium alloys suitable for preparation and production of castings for direct use through casting methods.
(2) Wrought magnesium alloys
Magnesium alloys that can be processed by plastic forming methods such as extrusion, rolling, forging, and stamping.
V. Tin and Tin Alloys
1. Tin (Sn)
(1) Deposits and smelting
Ore: Cassiterite (SnO₂). Preparation: First, concentrate is produced (tin content 60%-70% by mass). Smelting: Tin is reduced from oxygen in vertical or flame furnaces, then crude tin is further refined by liquation or electrolysis.
(2) Main properties
1) Physical properties.
The melting point is 232°C, and the density is 7.3kg/cm³.
2) Chemical properties.
Resistant to air, water, and many alkalis and acids.
3) Mechanical properties. Tensile strength is 30MPa, elongation ≤40%.
4) Processing properties.
Non-toxic, with good formability and ductility. Below -200°C, tin becomes brittle and fractures, and below -20°C it turns to powder. Tin is ductile and can be rolled, punched, and hammered. It can be made into tin foil with a thickness of less than 0.01mm.
2. Tin alloys
Tin alloys are alloys formed by adding other alloying elements (copper, antimony, lead, etc.) to tin as the base. Tin alloys have low melting points, low strength and hardness, good thermal conductivity, and low thermal expansion coefficients. They are resistant to atmospheric corrosion, have excellent anti-friction properties, and are easy to solder with steel, copper, aluminum, and their alloys. They are good soldering materials and bearing materials.
(1) Tin-based bearing alloys
Collectively known as Babbitt alloys along with lead-based bearing alloys. Antimony content is 3%-15% by mass, copper content is 3%-10% by mass. Antimony and copper are used to increase the strength and hardness of the alloy. They have a low coefficient of friction, good toughness, thermal conductivity, and corrosion resistance, mainly used for manufacturing sliding bearings.
(2) Tin solders
Mainly tin-lead alloys. Tin alloy with 38.1% lead by mass is commonly known as solder, with a melting point of about 183°C, used for soldering components in the electrical instrument industry, and sealing automobile radiators, heat exchangers, food and beverage containers.
(3) Tin alloy coatings
Utilizing the corrosion resistance of tin alloys, they are applied to the surface of various electrical components, providing both protection and decoration.
(4) Tin alloys
(Including lead-tin alloys and lead-free tin alloys) used to produce various exquisite alloy jewelry and crafts, such as rings, necklaces, bracelets, earrings, brooches, buttons, tie clips, hat ornaments, decorative crafts, alloy photo frames, religious emblems, miniature statues, souvenirs, etc.
3. Processing
Tin has good fluidity and casting properties in the molten state and can be used as a coating material (such as tinplate).
VI. Lead and Lead Alloys
1. Lead (Pb)
(1) Deposits and smelting
The most important lead ore is galena (PbS) and mixed ores. First, rich lead concentrate is produced, then lead is obtained through roasting and reduction, followed by refining to obtain pure lead.
(2) Main properties
1) Physical properties.
The melting point is 327.4°C, and the density is 11.34kg/cm³.
2) Chemical properties.
Has very good corrosion resistance, resistant to most acids but not to aqua regia, toxic.
3) Mechanical properties.
Low strength and hardness, poor elasticity, tensile strength is 15MPa, elongation ≤60%.
4) Processing properties.
Low deformation resistance, high deformability, suitable for cold forming. Lead is easy to braze, weld, and cast. It can be alloyed with other metals. Mainly used for making cap sheets, acid-resistant containers, lead-sheathed cables, sealing rings, lead shots, radiation protection plates, and sealing lead.
2. Lead alloys
Lead alloys are alloys composed of lead as the base with the addition of other elements. According to properties and uses, lead alloys can be classified into corrosion-resistant alloys, battery alloys, solder alloys, printing alloys, bearing alloys, and die alloys. Lead alloys are mainly used for chemical corrosion protection, radiation shielding, making battery plates and cable sheaths.


