When it comes to metals, pewter and tin might seem like close cousins, but they each have unique characteristics that set them apart. Have you ever wondered why some antique dishes are prized as pewter while others are simply tin? This article will unravel the intriguing differences between these two metals, shedding light on their distinct properties, uses, and safety considerations. You’ll discover how their compositions influence their physical and aesthetic properties, and why some applications favor one over the other. So, is pewter truly safer than tin? And what makes each metal suitable for different purposes? Let’s dive in and explore the fascinating world of pewter and tin.
Introduction to Pewter and Tin
Introduction to Pewter
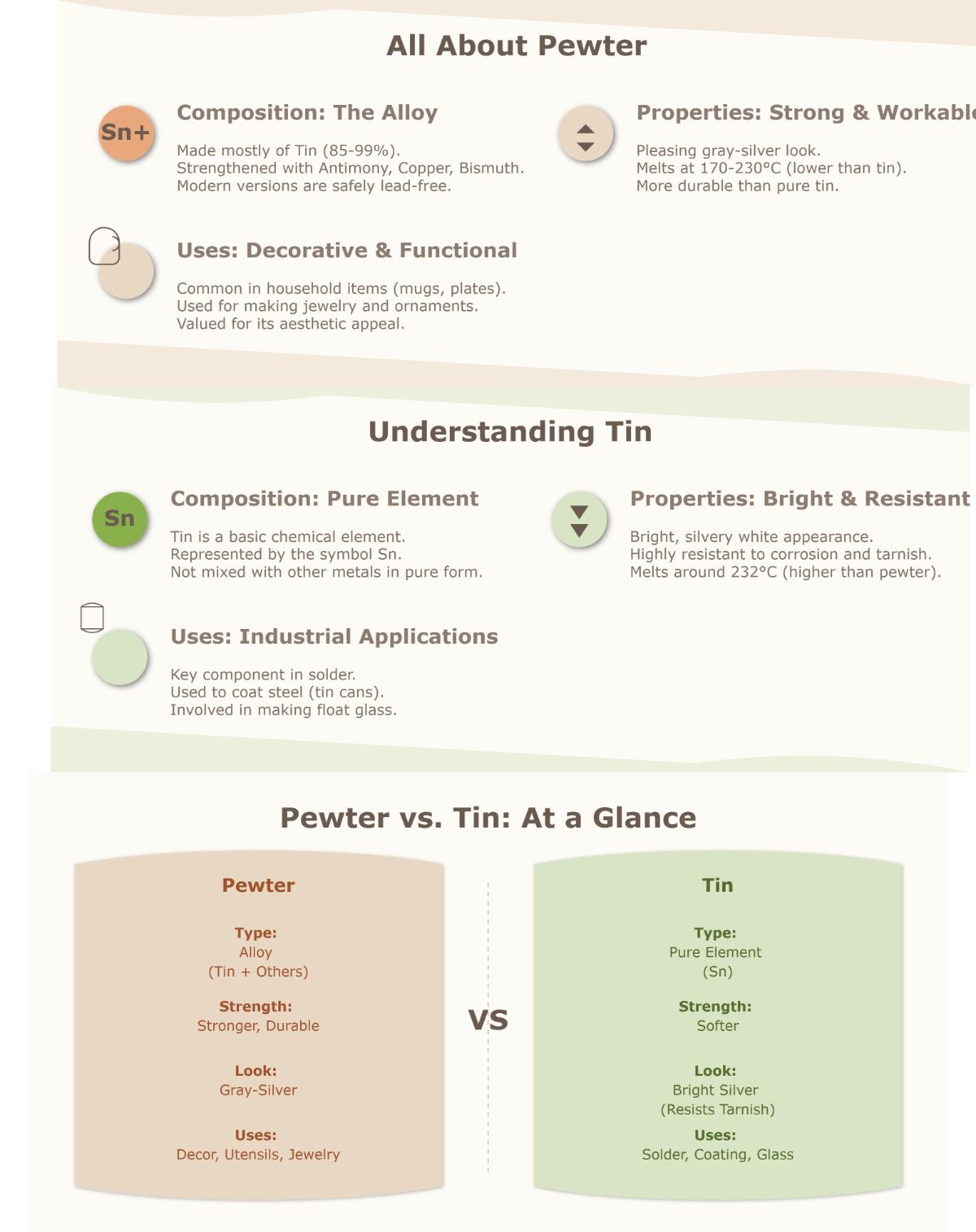
Pewter is an alloy primarily composed of tin, typically ranging from 85% to 99%. Historically, pewter contained lead to improve its malleability, but modern pewter is lead-free, making it safe for use with food and beverages. Pewter is known for its lustrous gray-silver appearance and is valued for its relatively low melting point, which varies between 170°C to 230°C (338°F to 446°F) depending on the specific alloy composition, making it excellent for crafting and decorative items.
Introduction to Tin
Tin is a pure chemical element with a bright silver-white color. Tin is characterized by its softness, malleability, and resistance to corrosion. It has a relatively low melting point of about 232°C (450°F), making it suitable for various industrial applications such as soldering, coating other metals to prevent rust, and the production of float glass. Tin’s purity and durability have made it a valuable material throughout history, significantly impacting metallurgy and trade.
Comparative Overview
Composition
- Pewter: Pewter is an alloy made mainly of tin (85% to 99%), with additional metals like antimony, copper, and sometimes bismuth or silver.
- Tin: Tin, on the other hand, is a pure element with no added metals.
Appearance
- Pewter: Lustrous gray-silver, often with a polished or matte finish.
- Tin: Bright silver-white, maintaining a shiny appearance over time.
Melting Point
- Pewter: Varies from 170°C to 230°C (338°F to 446°F) depending on alloy composition.
- Tin: Approximately 232°C (450°F).
Uses
- Pewter: Commonly used in crafting decorative items, jewelry, and household utensils, especially in applications requiring lead-free materials.
- Tin: Widely used in industrial applications such as soldering, coating steel to prevent corrosion, and in the production of float glass.
Both pewter and tin have unique properties that make them suitable for different applications. Pewter’s alloy composition allows for greater flexibility in crafting and decorative uses, while tin’s purity and corrosion resistance make it indispensable in various industrial processes. Understanding the fundamental differences between these materials helps in selecting the appropriate one for specific needs and applications.
Understanding Metal Alloys
Understanding Metal Alloys
Metal alloys are combinations of two or more metals, or a metal and another element, designed to enhance specific properties such as strength, durability, corrosion resistance, and workability. This section explores the concept of metal alloys and how they differ from pure metals, particularly focusing on pewter and tin.
What is an Alloy?
An alloy is a mixture that consists of a base metal combined with one or more additional elements. The base metal is the primary component of the alloy, while alloying elements are added to enhance specific properties. The purpose of creating alloys is to take advantage of the desirable properties of each constituent element, resulting in a material that has improved characteristics compared to its individual components.
Key Properties of Metal Alloys
Alloys are designed to have properties that pure metals might not possess. Some key properties include:
- Enhanced Strength: Alloys often have greater tensile strength than pure metals, making them more durable and suitable for heavy-duty applications.
- Improved Hardness: Alloying can increase the hardness of a material, making it more resistant to wear and deformation.
- Corrosion Resistance: Many alloys are designed to resist corrosion better than their base metals, extending their lifespan in harsh environments.
- Workability: Alloys can be easier to shape and form, which is beneficial in manufacturing processes.
Tin: A Pure Metal
- Definition: Tin is a chemical element with the symbol Sn. It is a soft, malleable, and silvery-white metal.
- Properties: Tin is known for its corrosion resistance and low toxicity. It melts at about 232°C (450°F) and is non-magnetic.
- Uses: Tin is widely used in soldering, coating other metals to prevent rust, and in the production of float glass.
Pewter: A Metal Alloy
- Definition: Pewter is an alloy primarily made of tin, typically comprising 85% to 99% tin. The remaining composition includes metals like antimony, copper, and sometimes bismuth or silver.
- Properties: The addition of these metals enhances pewter’s hardness, strength, and durability. Pewter has a lower melting point than pure tin, typically ranging from 170°C to 230°C (338°F to 446°F), depending on its exact composition.
- Uses: Pewter is commonly used for crafting decorative items, tableware, jewelry, and figurines. Modern pewter is lead-free, making it safe for food-related applications.
Comparative Analysis: Tin vs Pewter
| Feature | Tin (Pure Metal) | Pewter (Tin Alloy) |
|---|---|---|
| Composition | 100% tin | 85–99% tin + antimony, copper, bismuth, sometimes silver |
| Melting Point | ~232°C (450°F) | 170–230°C (338–446°F) (lower due to alloying metals) |
| Hardness & Strength | Soft and malleable | Harder and more durable due to alloying elements |
| Corrosion Resistance | Good | Often better due to alloying |
| Toxicity Concerns | Non-toxic | Lead-free and safe |
| Uses | Coating, solder, plating, and pure metal artifacts | Tableware, decorative objects, jewelry, figurines |
| Magnetic Properties | Non-magnetic | Non-magnetic |
Understanding the differences between pewter and tin highlights the advantages of using alloys. Pewter, as a tin alloy, leverages the beneficial properties of tin while incorporating other metals to improve its overall performance and suitability for various applications.
Composition of Pewter and Tin
Pewter is an alloy primarily made of tin, usually comprising 85% to 99% of its total composition. It often includes small amounts of other metals such as antimony, copper, and sometimes bismuth or silver to enhance its properties. Historically, pewter often included lead to improve its malleability, but modern pewter is lead-free due to health concerns. Typically, modern pewter consists of around 91% tin, 7.5% antimony, and 1.5% copper, making it safe for use in items related to food and drink.
Tin, symbolized as Sn, is a pure metal known for its silvery-white color, softness, and malleability. It is relatively unreactive and has a low melting point of around 232°C (450°F). This makes tin useful in various applications, such as soldering, coating other metals to prevent rust, and producing alloys like pewter.
Key Differences Between Pewter and Tin
Composition
- Tin: Composed almost entirely of tin.
- Pewter: An alloy primarily made of tin (85-99%) combined with antimony, copper, and sometimes bismuth or silver.
Hardness and Durability
- Tin: Soft and malleable, making it less ideal for high-durability applications.
- Pewter: Harder and more durable due to the addition of metals like antimony and copper, making it suitable for crafting and decorative items.
Appearance
- Tin: Silvery-white and shiny, maintaining a bright appearance over time.
- Pewter: Bluish-white with a bright or satin finish, depending on its specific composition and treatment.
Melting Point
- Tin: Melts at approximately 232°C (450°F).
- Pewter: Has a lower melting point, typically ranging from 170°C to 230°C (338°F to 446°F), depending on the alloy composition.
Toxicity
- Tin: Non-toxic and safe for most applications.
- Pewter: Modern lead-free pewter is safe for use, especially in items like dishes and drinking vessels. Historical pewter containing lead is toxic and not safe for food-related uses.
Corrosion Resistance
- Tin: Good resistance to corrosion, making it suitable for coating other metals.
- Pewter: Resists tarnish and corrosion well, often better than pure tin, due to the presence of alloying metals.
Uses
- Tin: Frequently used in industrial applications like soldering, steel coating, and float glass production.
- Pewter: Widely used for making domestic utensils, decorative items, jewelry, and cast figurines due to its attractive appearance and ease of shaping.
Properties of Pewter and Tin
Physical Properties
Density
Pewter and tin have distinct densities that influence their applications and handling. Pewter, being an alloy, has a density ranging from 8.5 to 9.5 g/cm³. This relatively high density gives pewter a substantial feel, which is often desirable in decorative and functional items. In contrast, pure tin has a slightly lower density of about 7.31 g/cm³, making it lighter than pewter.
Melting Point
The melting points of pewter and tin differ due to their compositions. Pewter has a melting point that typically ranges between 170°C and 230°C (338°F to 446°F), depending on the specific alloy mixture. This lower melting point makes pewter easy to cast and shape, which is advantageous for artisans and manufacturers. Tin, being a pure element, has a consistent melting point of approximately 232°C (450°F), which is slightly higher than most pewter alloys but still relatively low compared to many other metals.
Hardness
Pewter is generally softer than many other metals but harder than pure tin. The addition of antimony and copper in pewter alloys enhances its hardness and strength, making it more durable and resistant to deformation. Pure tin, on the other hand, is quite soft and malleable, which can limit its use in applications requiring structural integrity.
Aesthetic Properties
Appearance
Pewter has a lustrous gray-silver look that can be polished to a high shine or left with a matte finish, making it versatile for decorative items. This versatility makes pewter popular for both ornamental and functional uses. Tin, however, has a bright silver-white color that maintains its shine over time, providing a clean and reflective surface.
Finish
Pewter can be treated to achieve various finishes, including polished, satin, and antiqued looks, adding to its appeal for ornamental uses. This allows pewter items to fit various styles and preferences. Tin typically retains its bright, shiny finish, which is desirable for applications where a reflective and corrosion-resistant surface is needed.
Durability and Longevity
Wear Resistance
Pewter, due to its alloyed nature, exhibits good wear resistance, making it suitable for items that undergo frequent handling or use, such as tableware and jewelry. The presence of antimony and copper makes pewter more durable compared to pure tin. Tin, while corrosion-resistant, is softer and more prone to wear and deformation under mechanical stress, limiting its use in applications requiring high durability.
Lifespan
Both pewter and tin offer long lifespans, especially when properly maintained. Pewter’s resistance to tarnish and corrosion ensures that items made from it can remain attractive and functional for many years. Tin also boasts excellent corrosion resistance, contributing to its longevity, particularly in protective coatings and soldering applications where it prevents rust and degradation of underlying materials.
Chemical Properties
Corrosion Resistance
Pewter and tin both have good corrosion resistance, which is one of the reasons they are valued in various applications. Pewter’s alloy composition, particularly the inclusion of antimony and copper, enhances its ability to resist tarnishing and corrosion. This makes pewter suitable for decorative and functional items that may be exposed to the elements. Tin, known for its excellent corrosion resistance, is often used to coat other metals to prevent rust and corrosion, further extending the life of the items it protects.
Reactivity
Tin is relatively unreactive with most substances, contributing to its stability and durability in various environments. This low reactivity makes tin suitable for use in applications such as food packaging and coatings, where chemical stability is crucial. Pewter, while also relatively stable, may tarnish under certain conditions, such as exposure to sulfur compounds. However, pewter remains a durable choice for many applications due to its overall low reactivity.
Applications of Pewter and Tin
Pewter: Applications and Characteristics
Pewter, a malleable metal alloy primarily composed of tin, is known for its versatility and attractive finish. Its composition often includes small amounts of copper, antimony, and sometimes bismuth, which enhance its properties.
Decorative Objects
Pewter is extensively used to craft collectible items such as statuettes, figurines, replica coins, game figures, and pendants. Its fine castability and engravability make it perfect for detailed art and craft pieces.
Tableware and Drinkware
Due to its lustrous sheen and ability to retain heat, pewter is popular for creating plates, bowls, mugs, cups, teapots, and stemware. These items are often used for special dining or as heirloom pieces.
Jewelry
Pewter is a favored material in jewelry making, including earrings, necklaces, bracelets, and rings. Its affordability, light weight, and intricate design potential make it ideal for beautiful jewelry.
Home Decor
Pewter adds a touch of sophistication to home decor items like candlesticks, picture frames, vases, and ornaments. Its classic look makes it a popular choice for enhancing interior aesthetics.
Awards and Medals
Pewter is also used in crafting awards and medals, such as the fourth-place medals in the United States Figure Skating Championships. Its prestige and symbolic value make it suitable for such applications.
Crafts and Art Projects
The low melting point and ease of casting make pewter an excellent material for artists and hobbyists. It allows for the creation of custom reliefs, engravings, and cast pieces, fostering creativity in various art projects.
Recycling and Sustainability
Pewter can be recycled indefinitely without losing quality. Its low melting point allows for easy recasting, making it eco-friendly and cost-effective for artisans.
Tin: Applications and Characteristics
Tin, a pure chemical element, is known for its corrosion resistance, low toxicity, and good electrical conductivity. It is used in both pure form and as a component in various alloys, including pewter.
Industrial Uses
Tin’s corrosion resistance and electrical properties make it valuable in industries. It is widely used in electrical connectors, soldering, plumbing fixtures, and various other industrial components.
Coating Material
Tin is often used as a coating to prevent the corrosion of other metals. A common example is tin-plated steel cans used for food packaging, commonly known as tin cans.
Alloy Component
Tin plays a crucial role in forming alloys such as pewter and bronze. Its addition enhances the malleability and corrosion resistance of these alloys, making them more suitable for various applications.
Pewter vs. Tin: Key Differences in Application
| Feature | Pewter | Tin |
|---|---|---|
| Composition | Alloy primarily of tin with copper, antimony, etc. | Pure elemental metal |
| Appearance | Lustrous silvery-gray finish, good for decorative uses | Silvery-white, less lustrous |
| Common Uses | Decorative art, tableware, jewelry, medals, home decor | Industrial components, soldering, coatings, plating |
| Workability | Easily cast, engraved, low melting point | Used mostly in industrial forms, less decorative |
| Recyclability | Recycled easily with no quality loss, eco-friendly | Also recyclable but mainly industrial recycling |
| Durability | Durable for decorative use but softer than many metals | Resistant to corrosion, used to protect other metals |
| Cost and Availability | Affordable for artisans and craftspeople | Widely available, essential industrial metal |
Safety Considerations
Understanding the composition of pewter and tin is essential for ensuring their safe use, especially in food and drink applications.
Composition and Toxicity
- Tin is a pure metal historically used in food containers and packaging due to its non-toxic and corrosion-resistant nature, posing minimal health risks in solid form. However, tin powders can be combustible and reactive, necessitating careful handling during manufacturing or processing.
- Pewter is an alloy primarily composed of tin (about 95-97%) with additional metals such as copper and antimony. Modern pewter, used for food-related items, is lead-free or contains less than 0.05% lead, complying with strict regulations like those of the FDA. Lead-free pewter is non-toxic and considered safe for items such as tableware and jewelry.
Reactivity and Usage Safety
Evaluating the reactivity of pewter and tin with different substances is crucial for ensuring their safe use, particularly in contact with food.
- Tin is highly stable and durable, making it ideal for long-term food storage. It does not significantly react with acidic foods and is often used to coat other metals to enhance protection. This stability ensures that tin is a reliable and safe choice for surfaces that come into contact with food.
- Pewter, on the other hand, can react with acidic foods, which might cause the alloy to degrade or leach metals into the food. Due to its lower melting point and reactivity, pewter is not recommended for long-term storage of food or beverages, or for cooking applications such as in ovens or microwaves. Proper maintenance, such as thorough cleaning and drying after use, is necessary to prevent bacterial growth and avoid scratches that could expose underlying metals.
Health Concerns About Alloy Components
The health implications of the metals used in pewter alloys are important to consider.
- Antimony, used to harden pewter, is toxic at high levels, but the small amounts in lead-free pewter are generally safe for normal use and food contact.
- Lead-containing pewter was historically common but has largely been replaced by lead-free formulations due to the significant health risks associated with lead exposure, particularly in items related to food.
| Aspect | Tin | Pewter |
|---|---|---|
| Composition | Pure tin, sometimes coated | Alloy: ~95% tin + copper + antimony, lead-free versions predominant |
| Toxicity | Non-toxic, safe for food contact | Lead-free pewter is food-safe; older leaded pewter is unsafe |
| Reactivity | Stable, non-reactive with acidic foods | Can react with acidic foods, not for long-term storage or cooking |
| Durability | High, suitable for long-term food storage | Lower melting point, less durable for cooking or storage |
| Handling Precautions | Tin powder combustible, handle carefully | Clean and dry after use to prevent corrosion and bacterial growth |
Regulatory Standards
Adhering to regulatory standards ensures the safety and compliance of pewter and tin products.
- Lead-free Regulations: Modern pewter must comply with strict regulations to be considered lead-free, ensuring it is safe for use in food-related items.
- Compliance with Safety Standards: Both pewter and tin products should adhere to safety standards set by regulatory bodies like the FDA to ensure they are non-toxic and safe for consumer use.
Comparative Analysis
Pewter and tin have distinct compositions and properties that set them apart.
Composition
Pewter is an alloy primarily composed of tin (85-99%) with additional metals such as antimony, copper, and sometimes bismuth or silver. These additional metals enhance pewter’s strength and hardness. Tin, on the other hand, is a pure chemical element symbolized as Sn, with no added metals.
Pewter Composition
- Tin (85-99%)
- Antimony (5-10%)
- Copper (up to 2%)
- Bismuth/Silver (occasionally)
Tin Composition
- 100% Tin
Physical Properties
Density
Pewter has a higher density (8.5 to 9.5 g/cm³) due to its alloy composition, making it feel more substantial. In contrast, tin’s density is about 7.31 g/cm³, making it lighter.
Melting Point
Pewter’s melting point varies between 170°C and 230°C (338°F to 446°F), depending on the specific alloy mix. Tin has a consistent melting point of approximately 232°C (450°F).
Hardness
Pewter is harder and more durable than pure tin because of the added metals. Tin is softer and highly malleable, making it less suitable for applications requiring high strength.
Aesthetic Properties
Appearance
Pewter has a lustrous gray-silver appearance, which can be polished or left with a matte finish. Tin has a bright silver-white color that maintains its shiny appearance over time.
Finish
Pewter can achieve various finishes, including polished, satin, and antiqued looks, making it versatile for decorative items. Tin typically retains a bright, shiny finish, ideal for applications needing a reflective surface.
Applications
Pewter is popular for making decorative items, jewelry, utensils, and drinkware. Modern pewter, being lead-free, is safe for food and drinkware applications.
Tin is used extensively in industrial applications such as soldering, coating steel to prevent rust, and in the production of float glass. It is also used as an alloying element in various other metals.
Safety Considerations
Modern lead-free pewter is safe for food and drink items. Historically, pewter contained lead, which posed health risks.
Tin is non-toxic and safe for most applications, including those involving food contact. Its stability and low reactivity make it a reliable choice for long-term use.
Comparative Summary
| Aspect | Pewter | Tin |
|---|---|---|
| Nature | Alloy primarily of tin, with antimony and copper | Pure metallic element |
| Appearance | Gray-silver, lustrous | Bright silvery-white |
| Melting Point | 170–230 °C (depends on alloy composition) | ~232 °C |
| Density | 8.5 to 9.5 g/cm³ | 7.31 g/cm³ |
| Hardness | Harder and more durable | Softer, more malleable |
| Corrosion Resistance | Good | Good |
| Safety | Lead-free pewter is safe for food use | Non-toxic |
| Common Uses | Jewelry, utensils, decorative items | Soldering, coating, glass |
| Historical Use | Evolved from lead alloys to modern safe alloys | Ancient and widespread metal |
Frequently Asked Questions
Below are answers to some frequently asked questions:
What are the main differences between pewter and tin?
Pewter and tin differ primarily in their composition and properties. Pewter is an alloy predominantly composed of tin (85-99%), with additional elements like antimony, copper, and bismuth, which enhance its strength and durability. Historically, pewter included lead, but modern pewter is lead-free for safety reasons. Tin, on the other hand, is a pure element with a bright, silvery appearance and is known for its excellent corrosion resistance.
Physically, pewter has a lustrous gray-silver color and a density ranging from 8.5 to 9.5 g/cm³, with a melting point between 338°F to 446°F (170°C to 230°C). Tin has a slightly higher melting point of about 450°F (232°C) and maintains its brightness without tarnishing.
In terms of applications, pewter is commonly used for household utensils, jewelry, and decorative items due to its aesthetic appeal and workability. Tin is primarily used for soldering, coating steel, and manufacturing float glass.
Modern pewter is safer for use in food and drinkware as it is lead-free, whereas pure tin is valued for its resistance to tarnishing but lacks the enhanced strength of pewter. Overall, pewter’s alloy composition makes it more versatile and durable for a wider range of uses compared to pure tin.
Is pewter safer to use than tin?
Pewter and tin both have safety profiles that depend on their composition and usage. Tin is a chemical element that is naturally lead-free and non-toxic, making it safe for food and drink contact. It is stable and suitable for long-term storage, including acidic foods, due to its corrosion resistance and inert nature.
Pewter, on the other hand, is an alloy primarily made of tin (85–99%) with small amounts of other metals like antimony and copper. Modern pewter is usually lead-free or contains very minimal lead (less than 0.05%), making it safe for food and drink use when handled properly. However, pewter can react with acidic foods, potentially causing corrosion or taste changes, and has a low melting point, making it unsuitable for cooking or long-term storage of acidic foods.
What are typical applications of pewter and tin?
Pewter and tin have different typical applications due to their unique properties and compositions.
Pewter is widely used in decorative and craft items. It is popular for making collectible statuettes, figurines, aircraft models, replica coins, pendants, and plated jewelry. Its lustrous finish and durability make it ideal for these purposes. Additionally, pewter is used in tableware such as plates, bowls, mugs, cups, teapots, and stemware due to its heat-retaining properties and aesthetic appeal. Home decor items like candlesticks, picture frames, vases, and ornaments also frequently utilize pewter. In the industrial sector, pewter’s low melting point and casting properties make it suitable for electrical connectors and plumbing fixtures. Artists and hobbyists often choose pewter for relief work, engraving, and other creative projects.
Tin, on the other hand, is commonly used in soldering, where it forms strong bonds between metals. It is also prevalent in packaging, particularly in tin cans for food preservation. Tin is used in electrical wiring and as a protective coating for steel to prevent rusting, known as tin plating.
How do the environmental impacts of pewter and tin compare?
When comparing the environmental impacts of pewter and tin, it is important to understand their compositions and properties. Tin is a naturally occurring, non-toxic metal that is abundant and has a relatively low environmental impact from mining. It does not require intensive chemical treatments, making it an environmentally friendly option.
Pewter, on the other hand, is an alloy primarily made of tin but also includes small amounts of metals like copper and antimony. While modern pewter is lead-free and non-toxic, its environmental impact is slightly higher due to the extraction and processing of these additional metals, which can disrupt habitats and cause pollution.
However, pewter has a significant environmental advantage due to its high recyclability. It can be recycled indefinitely without losing quality, reducing the need for new raw material extraction. Additionally, pewter’s low melting point makes recycling and remolding more energy-efficient compared to other metals.
What are the benefits of using lead-free pewter?
Lead-free pewter offers significant benefits, especially when compared to traditional pewter containing lead. Firstly, it is non-toxic and safe for everyday use, including in items like jewelry and tableware that come into direct contact with skin and food. This eliminates the risk of lead poisoning associated with older pewter alloys. Secondly, lead-free pewter retains its color and resists tarnishing and corrosion, maintaining an attractive finish over time with minimal maintenance. Additionally, it is durable and robust, making it suitable for various applications without becoming brittle. Environmentally, lead-free pewter is more sustainable as it can be recycled repeatedly without losing quality. Finally, it is easy to work with, melting at low temperatures and allowing for detailed designs, which is advantageous for artisans and manufacturers. These benefits make lead-free pewter a preferred choice for modern metal goods.
Are there any health concerns associated with using pewter or tin items?
There are some health concerns associated with using pewter and tin items, primarily depending on their composition and usage. Modern pewter, which is an alloy primarily composed of tin, copper, and antimony, is usually lead-free and considered safe for food and drink use. The FDA approves lead-free pewter (containing less than 0.05% lead) for contact with food. However, older or antique pewter often contains high levels of lead, which can leach into food and beverages, posing significant health risks such as lead poisoning.
Tin, being a non-toxic metal, is generally safe for use in food and drink items. However, both pewter and tin should not be used with highly acidic or alkaline substances, as these can cause the metals to corrode and potentially leach harmful substances. Additionally, neither material is microwave-safe due to their metal content.
To ensure safety, it is crucial to verify that pewter items are modern and lead-free, and to use both pewter and tin items appropriately.
