Have you ever wondered how industries manage to separate valuable metals from intricate alloys? If you’ve ever found yourself curious about the meticulous process of separating copper and zinc alloys, you’re in the right place. This guide is tailored to provide a comprehensive, step-by-step walkthrough for intermediate learners keen on mastering the art of metal separation. We’ll explore various methods such as distillation and electrolysis, delving into their mechanisms and efficiency. Alongside these techniques, we’ll discuss the environmental benefits of recycling these metals, emphasizing sustainability. So, how exactly do these processes work, and which method is most effective for your needs? Let’s dive in and uncover the secrets to efficiently separating copper and zinc.
Introduction to Copper and Zinc Alloys
Copper and zinc alloys, known as brass, are highly adaptable and widely used in various industries. These alloys are formed by combining copper and zinc in different proportions, allowing for the adjustment of properties such as strength, ductility, and corrosion resistance.
The composition of brass significantly influences its mechanical properties. Low zinc content (up to 37%) results in the α-phase, enhancing tensile strength and elongation. Intermediate zinc content (37% to 46%) introduces an α+β-phase structure, increasing hardness and tensile strength while reducing plasticity. High zinc content (above 46%) leads to the β-phase, which increases hardness but reduces elongation. These phase changes are crucial for tailoring brass to meet specific industrial requirements.
Higher zinc content in brass decreases density, thermal conductivity, and the modulus of elasticity, while increasing the linear coefficient of thermal expansion. These changes allow manufacturers to select the right brass composition for specific applications.
Brass is highly recyclable, reducing the need for virgin materials and minimizing greenhouse gas emissions during production. Its durability and corrosion resistance extend the lifespan of products, contributing to sustainability.
The versatility of brass makes it suitable for a wide range of applications, including plumbing and electrical systems, the automotive industry, architecture and decoration, medical devices, household fixtures, and coinage. Its excellent corrosion resistance, strength, durability, and aesthetic appeal make it an ideal choice for these uses.
To enhance the machinability of brass, elements like lead, tin, silicon, aluminum, and phosphorus can be added. These additions act as chip breakers, refine crystal grains, and distribute the gamma phase uniformly, ensuring efficient handling of brass components. Optimizing machining processes with appropriate tools and coolants further reduces overheating and improves production quality.
Methods for Separating Copper and Zinc
Separating copper and zinc from their alloys involves selecting the right method based on cost, efficiency, environmental impact, and desired metal purity. Each method comes with its own set of advantages and challenges, making it essential to choose the most suitable approach for your specific needs.
Chemical Processes
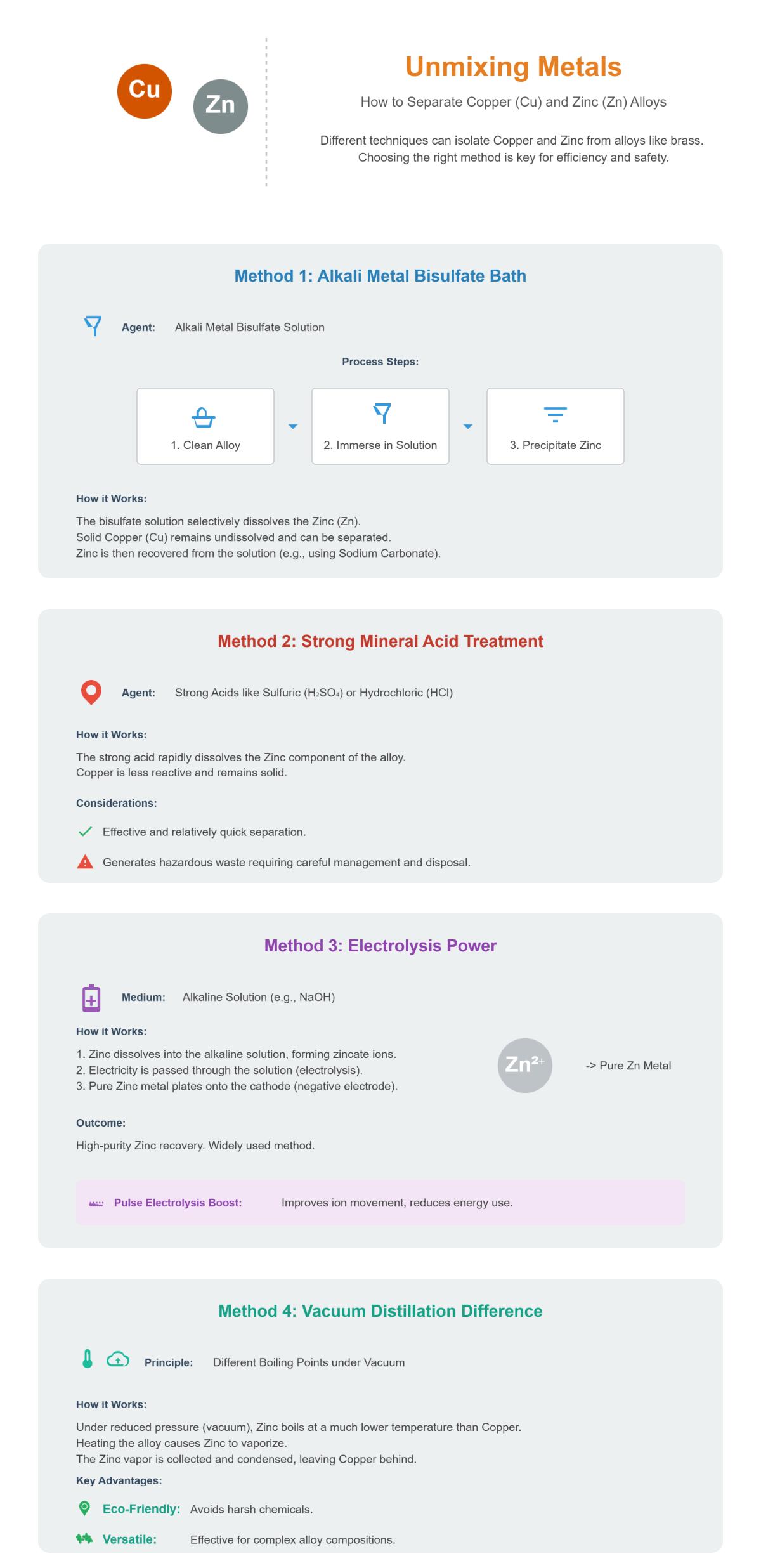
Alkali Metal Bisulfate Method
This technique involves immersing the copper-zinc alloy in an aqueous solution of alkali metal bisulfate. The chemical reaction selectively dissolves zinc, leaving copper behind. The process begins with preparing the solution by dissolving alkali metal bisulfate in water. The alloy is then submerged in this solution, where the zinc reacts and dissolves. Finally, reagents like sodium carbonate are added to precipitate zinc from the solution. While effective, this method requires careful handling of chemicals and responsible waste management.
Strong Mineral Acids
Using strong mineral acids such as sulfuric or hydrochloric acid is another approach. These acids can selectively dissolve zinc, leaving copper intact. The process involves preparing the acid to a suitable concentration, immersing the alloy in the acid solution to dissolve zinc, and then neutralizing the resulting hazardous waste for safe disposal. Although effective, this method produces waste that must be managed properly.
Ammoniacal Solutions
Ammoniacal solutions offer a selective approach for zinc dissolution, particularly useful for specific alloy compositions. This method can be advantageous when precise separation is required.
Electrolysis
Electrolysis provides a highly effective means of separating metals, achieving high purity with minimal cross-contamination.
Pulse Electrolysis
Pulse electrolysis combines alternating current pulses with alkaline leaching to dissolve zinc, leaving copper as a solid. The process involves dissolving zinc using an alkaline solution, then applying alternating current pulses during electrolysis to improve the separation of metals. This technique is especially beneficial in recycling and refining metals, offering high-purity separation.
Heat-Based Techniques
Vacuum Distillation
Vacuum distillation involves heating the alloy under reduced pressure, allowing zinc to vaporize and separate from copper because it has a lower boiling point than copper. This process includes heating the alloy in a vacuum environment, vaporizing the zinc, and then condensing it to collect separately. Although energy-intensive, this method is effective for achieving high-purity separation.
High Temperature Distillation
High temperature distillation requires significant capital investment but can separate zinc from copper in a single step. It operates at higher temperatures and pressures, providing an efficient method for metal separation.
Mechanical Methods
Mechanical separation methods, though less common for copper and zinc, can include grinding and sorting based on physical properties. These methods are typically less effective for mixed alloys but can be useful in preliminary separation stages.
Distillation Process
Explanation of Distillation
Distillation separates components in a mixture based on their different boiling points. In the context of separating copper and zinc alloys, distillation takes advantage of the significantly lower boiling point of zinc compared to copper. This method is particularly effective for achieving high-purity separation of these metals.
How Distillation Is Used to Separate Copper and Zinc
Distillation involves heating the copper-zinc alloy to a temperature where zinc vaporizes but copper remains in a solid or molten state. The zinc vapor is then condensed and collected separately, resulting in the separation of the two metals. Here’s a detailed step-by-step guide on how this process is carried out:
Equipment Needed
- Vacuum Furnace: Essential for maintaining a controlled environment during the process.
- Vacuum Pump: Reduces pressure to lower the boiling points of metals.
- Protective Gas Setup: Prevents oxidation by maintaining an inert atmosphere.
- Cooling System: Manages heat and condenses metal vapors.
- Electric Control Panel: Monitors and controls the entire process.
Step-by-Step Distillation Process
Preparation
- Cleaning the Alloy: Clean the copper-zinc alloy thoroughly to remove impurities that may affect the boiling points and contaminate the process.
Melting and Placement
- Melting the Alloy: Melt the copper-zinc alloy using a high-temperature furnace. Ensure the alloy is completely molten to facilitate uniform heating during distillation.
- Placement in Furnace: Place the molten alloy into the vacuum distillation furnace. Ensure the furnace is preheated to the desired temperature.
Vacuum Conditions
- Creating Vacuum Conditions: Turn on the vacuum pump to lower the furnace pressure, which reduces the boiling points of the metals, especially zinc.
Vaporization and Condensation
- Vaporization of Zinc: As the temperature increases, zinc vaporizes due to its lower boiling point. The vaporized zinc travels to a cooler section of the system.
- Condensation: Zinc vapor is condensed back into liquid or solid zinc, depending on the cooling temperature and environment. The cooling system ensures efficient condensation and collection of zinc.
Collection of Pure Metals
- Collecting Zinc: The condensed zinc is collected separately from the remaining solid copper. This step completes the separation process, resulting in pure zinc and copper.
Post-Process Treatment
- Further Refining: Depending on the intended use, the collected metals might need further refining to increase purity. This could involve additional distillation or chemical treatments.
Advantages and Limitations of Distillation
Advantages
- High Purity: Distillation produces high-purity zinc and copper, essential for various industrial applications.
- Efficiency: Operating under vacuum conditions reduces the temperature required for metal vaporization, making the process more energy-efficient.
- Environmental Benefits: Distillation generates minimal waste and emissions, supporting sustainable metal processing practices.
Limitations
- Energy-Intensive: The process requires significant energy input, especially for maintaining high temperatures and vacuum conditions.
- Equipment Costs: The initial capital investment in vacuum furnaces and related equipment can be substantial.
- Complexity: The process requires precise control and monitoring to ensure efficient separation and high purity.
Electrolysis Process
Understanding Electrolysis
Electrolysis is an electrochemical process that uses an electric current to drive a non-spontaneous chemical reaction. This process is crucial for separating and purifying metals, such as copper and zinc from their alloys. During electrolysis, metal ions are dissolved in an electrolyte solution and then deposited onto an electrode.
Steps for Separating Copper and Zinc Using Electrolysis
Preparation of the Alloy
- Cleaning and Shaping: Start by cleaning the copper-zinc alloy to remove contaminants, then shape it into thin sheets or rods to maximize surface area and enhance the efficiency of the electrolysis process.
Electrolyte Preparation
- Selection of Electrolyte: Choose an appropriate electrolyte that can selectively dissolve one of the metals. A copper(II) sulfate solution is often used for this purpose.
- Concentration: Prepare the electrolyte at the right concentration to ensure efficient metal ion dissolution and deposition.
Setup of the Electrolysis Cell
- Anode and Cathode Placement: The impure copper-zinc alloy is used as the anode, where oxidation occurs. Pure copper is typically used as the cathode, where reduction and deposition of copper ions occur. Arrange these electrodes properly in the electrolysis cell.
- Electrolysis Conditions: Set the electrolysis conditions, including temperature, current density, and electrolyte concentration. These parameters must be controlled precisely to optimize the separation process and ensure high purity of the separated metals.
Electrolysis Process
- Oxidation at the Anode: During electrolysis, zinc and other impurities are oxidized at the anode. Zinc, which has a more negative standard electrode potential than copper, oxidizes first and dissolves into the electrolyte.
- Reduction at the Cathode: Copper ions in the electrolyte are reduced at the cathode and deposited as pure copper. This step results in the gradual purification of copper on the cathode while zinc remains in the solution.
Recovery of Zinc
- Precipitation of Zinc: Add sodium carbonate to the solution to precipitate zinc as zinc carbonate, which can then be filtered and collected.
- Purification of Zinc: The precipitated zinc carbonate can be further processed to obtain pure zinc. This may involve additional chemical treatments or thermal processes to convert zinc carbonate into metallic zinc.
Benefits and Challenges of Using Electrolysis
Benefits
- High Purity: Electrolysis can achieve high purity levels for separated metals, making it suitable for applications requiring high-quality copper and zinc.
- Selective Separation: The process allows for selective separation of metals based on their electrochemical properties, reducing the likelihood of cross-contamination.
Challenges
- Energy Consumption: Electrolysis can be energy-intensive, particularly for large-scale operations, which may impact the overall cost-effectiveness.
- Process Control: Maintaining precise control over electrolysis conditions is crucial for optimizing efficiency and purity, requiring specialized equipment and expertise.
By following these steps and understanding the underlying principles of electrolysis, it is possible to effectively separate and purify copper and zinc from their alloys.
Other Separation Techniques
Vacuum Distillation
Vacuum distillation uses reduced pressure to separate metals based on their different boiling points. This method is particularly effective for separating zinc from copper due to zinc’s significantly lower boiling point.
Process of Vacuum Distillation
- Preparation and Vacuum Creation: Clean the copper-zinc alloy to remove impurities. Place the alloy in a vacuum furnace and use a vacuum pump to reduce the pressure. This lowers the metals’ boiling points.
- Heating and Vaporization: Gradually heat the alloy. Zinc, with its lower boiling point, will vaporize while copper remains in a solid or molten state.
- Condensation and Collection: Direct the zinc vapor to a cooler section where it condenses back into a liquid or solid form. The zinc is collected separately, leaving the copper behind.
Advantages of Vacuum Distillation
- High Purity: Produces high-purity metals due to effective separation.
- Environmental Benefits: Generates minimal waste and emissions.
Limitations
- Energy-Intensive: Needs a lot of energy to keep high temperatures and a vacuum.
- Equipment Costs: High initial investment in specialized vacuum furnaces and pumps.
Chemical Separation Using Alkali Metal Bisulfate
This method uses an aqueous solution of an alkali metal bisulfate, such as sodium bisulfate, to selectively dissolve zinc from the alloy.
Step-by-Step Procedure
- Preparation: Clean the alloy to remove impurities.
- Immersion and Dissolution: Immerse the alloy in an aqueous solution of alkali metal bisulfate at moderate temperatures (60°F to 120°F). Zinc reacts with the bisulfate and dissolves, while copper remains undissolved.
- Recovery: Add sodium carbonate to the solution to precipitate zinc as zinc carbonate or zinc hydroxide.
- Collection: Collect the undissolved copper, wash it thoroughly, and prepare it for further refinement.
Benefits
- Selective Separation: Efficiently separates zinc without affecting copper.
- Moderate Conditions: Operates under moderate temperatures and minimal aeration.
Challenges
- Chemical Handling: Requires careful handling of chemicals and proper waste management.
Strong Mineral Acids Separation
Using strong acids like sulfuric or hydrochloric acid can selectively dissolve zinc, leaving copper intact.
Methodology
- Preparation: Prepare a solution of the strong acid at a suitable concentration.
- Immersion and Dissolution: Submerge the alloy in the acid solution. Zinc dissolves into the acid, while copper remains unaffected.
- Neutralization: Neutralize the acidic waste for safe disposal.
Advantages
- Efficiency: Provides a quick and effective separation process.
Drawbacks
- Hazardous Waste: Generates hazardous waste that must be carefully neutralized and disposed of.
Ammoniacal Solutions
Ammoniacal solutions offer a selective approach for zinc dissolution, especially useful for specific alloy compositions.
Process
- Preparation: Clean the alloy to remove impurities.
- Immersion and Dissolution: Immerse the alloy in an ammoniacal solution. Zinc dissolves in the ammoniacal solution, leaving copper behind.
- Recovery: Recover and further process the dissolved zinc.
Safety Precautions and Best Practices
Personal Protective Equipment (PPE)
Separating copper and zinc alloys safely requires using proper personal protective equipment (PPE). Here’s a breakdown of the necessary PPE:
- Heat-resistant gear: Wear heat-resistant gloves, face shields, and aprons to protect against burns from molten metal and hot surfaces.
- Eye protection: Use safety glasses to guard against splashes, flying particles, and chemical exposure during processes such as grinding, welding, or handling acids.
- Respiratory masks: Employ NIOSH-approved respirators to avoid inhaling harmful fumes, such as zinc oxide, which can cause metal fume fever, characterized by flu-like symptoms.
Workspace Preparation
A well-prepared workspace is crucial for safe and efficient separation of copper and zinc alloys. Follow these guidelines to ensure a safe working environment:
- Ventilation: Ensure adequate airflow to disperse hazardous fumes, such as hydrogen gas released during acid reactions. This can be achieved through the use of fume hoods or exhaust fans.
- Spill management: Keep neutralizing agents like sodium bicarbonate readily available to manage acid spills. Store all chemicals in sealed containers to prevent accidental exposure.
- Tool safety: Use non-sparking tools and explosion-proof equipment to mitigate the risk of ignition from flammable gases.
Chemical Handling Protocols
Handling chemicals correctly is crucial for preventing accidents and ensuring effective separation. Adhere to the following protocols:
- Acid neutralization: After using strong acids (such as sulfuric or hydrochloric acid), neutralize them with bases to minimize hazardous waste and facilitate safe disposal.
- Bisulfate method: When using alkali metal bisulfate (e.g., sodium bisulfate) for selective zinc dissolution, maintain the solution temperature between 60–120°F to optimize the reaction.
- Metal displacement: To precipitate contaminants like lead and iron, add zinc metal to the bisulfate solution after zinc dissolution. This displacement reaction helps in purifying the solution.
Alloy Processing Steps
Processing alloys involves several critical steps to ensure effective separation and high-purity recovery of metals:
- Surface cleaning: Thoroughly clean the alloy to remove impurities such as oils and oxides, which can interfere with chemical reactions.
- Selective dissolution: Use the chosen chemical method (e.g., bisulfate or acid) to dissolve zinc while leaving copper undissolved.
- Zinc recovery: Precipitate dissolved zinc as zinc carbonate or hydroxide by adding sodium carbonate to the solution.
- Copper refinement: Purify the residual copper through electrolytic processes to achieve high-purity recovery.
Environmental and Health Considerations
Be mindful of the environmental and health impacts of the separation process. Take measures to mitigate these effects:
- Waste disposal: Neutralize acidic and alkaline effluents before disposal to prevent contamination of soil and water sources.
- Allergen management: Test alloys for trace nickel content to avoid dermatitis in sensitive individuals.
- Recycling benefits: Prioritize the use of recycled copper to reduce energy consumption by 85–90% compared to mining new copper, contributing to environmental sustainability.
Emergency Response
Preparation for emergencies can greatly reduce accident impacts:
- Metal fume exposure: If symptoms like fever or coughing occur due to metal fume exposure, seek immediate medical assistance.
- Chemical burns: In the event of chemical burns, rinse the affected area with water for at least 15 minutes and consult a physician promptly.
- Fire hazards: Use Class D fire extinguishers for metal fires and avoid using water, which can exacerbate the reaction.
Environmental Benefits of Recycling Alloys
Energy Conservation
Recycling copper-zinc alloys, such as brass, significantly reduces energy consumption compared to producing these metals from raw materials. Recycling processes use 85-90% less energy than the energy-intensive extraction and refinement of virgin metals, leading to lower carbon emissions and a smaller carbon footprint.
Resource Preservation
Recycling these alloys helps protect ecosystems and preserve natural resources by reducing the need for mining, which can cause environmental damage. Mining operations often result in habitat destruction, soil erosion, and water contamination. By recycling, we can minimize these harmful impacts, ensuring that natural resources remain available for future generations.
Pollution Reduction
Recycling copper-zinc alloys significantly reduces pollution by keeping scrap metal out of landfills, preventing heavy metals from contaminating soil and water. Additionally, recycling decreases the need for smelting ores, a process that releases greenhouse gases and other pollutants into the atmosphere. This leads to cleaner air and water, benefiting both the environment and public health.
Circular Economy Support
Recycling supports a circular economy by keeping metals in use indefinitely, reducing waste, stabilizing supply chains, and promoting sustainable industrial practices. In industries like electronics and construction, the continuous reuse of metals ensures a steady supply of raw materials, reducing dependency on new mining activities. This not only conserves resources but also drives innovation in recycling technologies and processes.
Compliance with Environmental Regulations
Recycling copper and zinc alloys helps industries comply with stringent environmental regulations. Governments and regulatory bodies worldwide are increasingly imposing laws to limit waste and reduce carbon emissions. By adopting recycling practices, companies can meet these regulatory requirements, avoid penalties, and enhance their reputation as environmentally responsible entities. Compliance also opens up opportunities for businesses to benefit from incentives and subsidies designed to promote sustainable practices.
Practical Applications of Separated Copper and Zinc
Applications of Separated Copper
Electronics and Electrical Applications
High-purity copper, obtained through separation processes, is extensively used in the electronics industry due to its excellent electrical conductivity. Its remarkable thermal conductivity also aids in dissipating heat, which is crucial for maintaining the performance and longevity of electronic devices.
Renewable Energy Systems
In renewable energy applications, copper’s superior electrical and thermal properties are essential, making it ideal for manufacturing wind turbine wiring and photovoltaic cells. The efficient transmission of electricity and the durability of copper contribute to the overall efficiency and reliability of these renewable energy systems.
Plumbing and Heating Systems
Copper is ideal for plumbing and heating systems because it resists corrosion and has antimicrobial properties. Separated copper is used in pipes, fittings, and heat exchangers, ensuring long-lasting and hygienic solutions for residential and industrial water systems.
Applications of Separated Zinc
Galvanization
Zinc is primarily used in the galvanization process, which involves coating steel or iron with a layer of zinc to prevent rusting. Electrolytically refined zinc provides a uniform and durable coating, protecting structures like bridges, automotive parts, and construction materials from corrosion.
Battery Production
Zinc is a critical component in various types of batteries, including zinc-air and zinc-carbon batteries. These batteries are used in a range of applications from grid storage solutions to medical devices like hearing aids, benefiting from zinc’s electrochemical properties and energy density.
Chemical and Pharmaceutical Industries
Zinc oxide, derived from separated zinc, is widely used in the chemical industry for the production of rubber, paints, and ceramics. In the pharmaceutical industry, zinc oxide is a key ingredient in sunscreens, ointments, and dietary supplements, owing to its protective and healing properties.
Industrial Benefits
Recycling and Resource Efficiency
Recycling copper and zinc from alloys like brass conserves natural resources and significantly reduces energy consumption. Recovered copper and zinc can be reintroduced into manufacturing processes, thereby reducing the need for virgin materials and lowering the environmental impact associated with mining and refining.
Cost-Effective Manufacturing
Using separated metals in manufacturing can lead to cost savings. High-purity copper and zinc reduce the need for additional refining, lowering production costs. Furthermore, the enhanced properties of these metals improve the quality and performance of the final products, contributing to economic efficiency.
Sustainability and Environmental Impact
Recycling and reusing copper and zinc support sustainable industrial practices by reducing waste and emissions. The energy efficiency of recycling processes compared to primary production helps in minimizing the carbon footprint, aligning with global efforts to combat climate change and promote a circular economy.
By leveraging the unique properties of separated copper and zinc, industries can enhance product performance, achieve cost savings, and contribute to environmental sustainability.
Frequently Asked Questions
Below are answers to some frequently asked questions:
What are the different methods for separating copper and zinc alloys?
Separating copper and zinc alloys, such as brass, can be achieved through several methods, each suited to specific applications and scalability. One common method is chemical separation using alkali metal bisulfate, where zinc selectively dissolves in the bisulfate solution while copper remains intact. This process involves cleaning the alloy, immersing it in the bisulfate solution, and then recovering zinc through precipitation with sodium carbonate.
Another method involves strong mineral acids like sulfuric or hydrochloric acid, which selectively dissolve zinc. This method is effective but requires careful handling of hazardous waste.
Electrolysis is also widely used, where an alkaline solution like NaOH dissolves zinc, and the zinc-rich solution undergoes electrolysis to recover high-purity zinc. Pulse electrolysis further enhances this process by improving ion mobility and reducing energy consumption.
Vacuum distillation exploits the differing boiling points of copper and zinc under reduced pressure to separate them. This method is environmentally friendly and effective for complex materials.
These methods each have specific advantages and challenges, making it crucial to choose the right approach based on the specific requirements of the separation process.
How does electrolysis help in separating copper and zinc?
Electrolysis aids in separating copper and zinc by exploiting their distinct electrochemical properties. The process begins with preparing the alloy, often involving cleaning and alkaline leaching to create a zinc-rich solution. During electrolysis, an electrolytic cell is set up with appropriate electrodes, and the zinc-rich solution serves as the electrolyte. By applying a controlled electrical current, zinc ions migrate to the cathode due to their lower reduction potential, where they are deposited as pure zinc. Meanwhile, the remaining copper is collected from the anode compartment or the residue. This method ensures high-purity separation, is energy efficient, and supports sustainable recycling practices. Recent advancements, like pulse electrolysis, further enhance efficiency by improving ion mobility and reducing energy consumption.
What are the environmental benefits of recycling copper and zinc alloys?
Recycling copper and zinc alloys offers significant environmental benefits. Primarily, it enhances energy efficiency since recycling these metals requires considerably less energy compared to their primary production. For instance, recycling copper can consume as little as 15% of the energy needed for mining and processing copper ore, leading to substantial reductions in greenhouse gas emissions. Additionally, recycling helps conserve natural resources by reducing the demand for new mining operations, thus preserving these valuable metals for future use.
Another key benefit is pollution reduction. Recycling copper and zinc minimizes the release of toxic substances that typically occur during primary extraction and processing. This not only protects the environment but also supports overall public health. Furthermore, recycling supports a circular economy by reintroducing materials into the production cycle, reducing waste, and lowering the need for landfill space. These practices contribute to sustainable resource management and provide economic advantages through cost savings and job creation in the recycling industry.
What practical challenges can arise during the separation process?
During the separation of copper and zinc alloys, several practical challenges can arise. Firstly, the similarity in physical and chemical properties, such as close melting points and reactivity, complicates traditional separation methods like smelting. Modern techniques like chemical processes or advanced thermal methods are required to address this. Impurities in the alloys further complicate separation, necessitating advanced technologies like membrane separation or AI optimization for effective impurity management. Energy consumption and environmental impact pose additional challenges, as conventional methods are energy-intensive and polluting. Sustainable methods, such as vacuum distillation and green chemical processes, offer solutions to reduce these impacts. Efficient recovery of both metals is crucial to minimize waste and pollution, with techniques like selective dissolution of zinc using alkali metal bisulfate solutions being effective. Lastly, ensuring scalability and cost-effectiveness is essential, requiring investment in innovative technologies that balance efficiency with economic and ecological benefits.
How can I troubleshoot common issues in the separation process?
To troubleshoot common issues in separating copper and zinc alloys, follow these steps:
- Chemical Separation: If using an alkali metal bisulfate solution, ensure the reaction time is sufficient and the bisulfate concentration is adequate to dissolve zinc completely. If copper contaminates the zinc solution, reduce aeration and maintain moderate temperatures to prevent copper dissolution. Adjust the pH during precipitation with sodium carbonate to avoid issues.
- Electrolysis: For low-purity recovered zinc, use high-purity electrolytes and extend electrolysis duration if needed. To prevent copper contamination in the zinc deposit, optimize the electrolyte composition and adjust pulse parameters. Improve cell design to reduce resistance and enhance energy efficiency.
- Strong Mineral Acids: When using acids like sulfuric or hydrochloric acid, ensure proper neutralization and disposal procedures to handle hazardous waste. Increase acid concentration and reaction time for complete zinc dissolution.
- Ammoniacal Solutions: Monitor and adjust the pH and ammonium ion concentrations to control solution chemistry effectively.
Additionally, maintain regular equipment maintenance, monitor the purity of recovered metals, and adhere to safety protocols when handling chemicals and electrical equipment. These steps will help optimize the separation process and ensure efficient, safe operations.
What safety precautions should be taken during the separation of copper and zinc alloys?
When separating copper and zinc alloys, several safety precautions are essential to ensure the protection of workers and the environment.
First, workers should be equipped with appropriate protective gear, including heat-resistant gloves, face shields, and respirators to protect against extreme temperatures, dust, and harmful gases. Adequate ventilation systems must be in place to control airborne dust and prevent inhalation of harmful particles. Regular monitoring of air quality and adherence to occupational exposure limits is crucial.
Proper handling and disposal of chemicals, such as alkali metal bisulfate, must be followed to avoid environmental contamination. Fire safety measures should include the use of self-contained breathing apparatuses and full protective clothing, along with clear emergency procedures.
Health precautions include immediate first aid for any exposure to copper and zinc in powder or smog form, such as flushing eyes with water or cleaning skin thoroughly. Sustainable practices, like recycling and using renewable energy sources, should be prioritized to minimize environmental impact. By adhering to these guidelines, a safe and responsible separation process can be maintained.
