When it comes to wiring in your home or business, understanding the intricacies of connecting different types of wires is crucial. Directly connecting copper and aluminum wires can lead to a host of problems, including galvanic corrosion, which compromises the safety and efficiency of your electrical system. But why does this happen, and how can you safely connect these two metals without risking electrical failures or hazards?
In this article, we’ll delve into the specific risks associated with copper and aluminum wire connections, such as the faster oxidation rate of aluminum and the thermal expansion mismatch that can cause loose connections and overheating. We’ll also explore practical solutions, including the use of specialized connectors and anti-oxidation compounds, to ensure safe and reliable electrical connections. By the end, you’ll have a comprehensive understanding of how to manage these connections effectively and keep your electrical systems running smoothly. Ready to learn more about safeguarding your wiring? Let’s dive in.
Introduction to Copper and Aluminum Wire Connections
Choosing the right wiring material is crucial for the safety, efficiency, and longevity of electrical systems. Copper and aluminum are two prevalent choices, each offering unique advantages and challenges. Copper excels in electrical conductivity and durability, resisting corrosion and bending easily. It efficiently handles high electrical loads but comes with a higher cost and weight. Aluminum, meanwhile, is lighter and less expensive, requiring a larger cross-sectional area to match copper’s current capacity. This necessitates larger conduits, which can impact installation design.
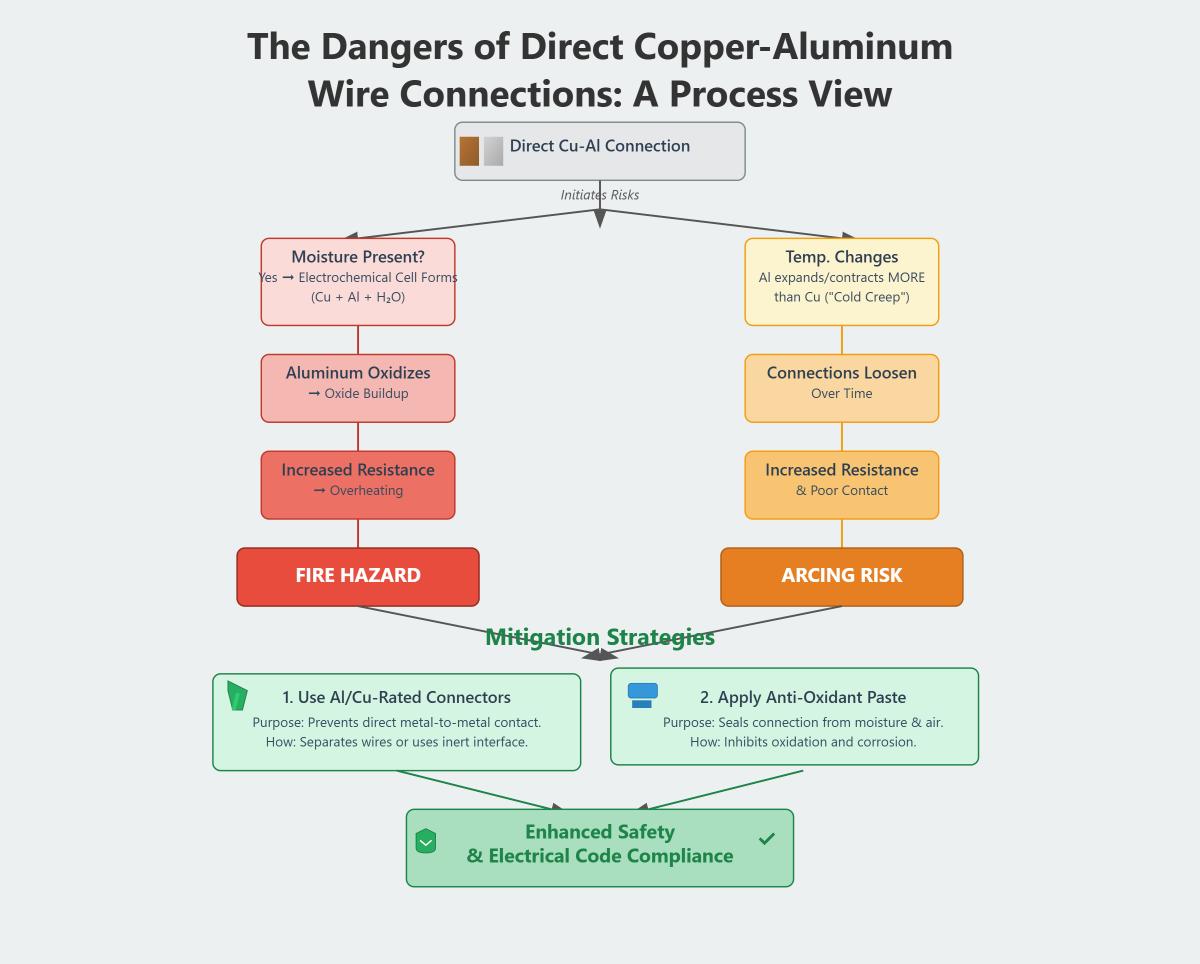
When connecting copper and aluminum wires, specific risks arise. A chemical reaction can occur when these metals contact each other in moist environments, potentially leading to increased resistance and overheating. Aluminum’s susceptibility to oxidation can form a non-conductive layer, further raising resistance and heat levels. Additionally, aluminum expands more with temperature changes than copper, risking loose connections and electrical failures over time.
To ensure safe connections, several strategies can be employed:
- Specialized Connectors: Use connectors like AlumiConn to prevent direct metal contact and reduce corrosion risks.
- Anti-Oxidation Compounds: Apply these compounds to aluminum wire connections to prevent non-conductive oxide layers.
- Proper Terminal Preparations: It’s essential to keep all connection points clean and free from oxidation, ensuring stable connections.
When mixing copper and aluminum wires, always opt for devices rated for both types, ensuring compatibility and safety. Using connectors designed for mixed wire types helps secure stable connections, minimizing corrosion and temperature-related issues. By applying these techniques, electrical systems can maintain their integrity and performance over time.
Galvanic Corrosion
Definition and Explanation
Galvanic corrosion is a type of electrochemical corrosion that occurs when two different metals, such as copper and aluminum, come into electrical contact within an electrolyte, often moisture. This phenomenon is driven by the difference in electrical properties between the metals. The less noble metal, aluminum, acts as the anode and corrodes to protect the more noble metal, copper, which acts as the cathode. Moisture accelerates galvanic corrosion, especially in humid or damp environments, which are common in outdoor electrical installations and HVAC systems.
Causes and Effects in Electrical Systems
Galvanic corrosion happens due to the electrochemical reaction between different metals. When copper and aluminum are connected, aluminum corrodes faster because it is more anodic. Moisture or condensation acts as an electrolyte, facilitating this corrosion by enabling the flow of electric current between the metals. Environmental factors such as humidity, poor ventilation, and exposure to elements increase the rate of corrosion by maintaining the presence of electrolytes. As a result, galvanic corrosion can increase electrical resistance, degrade materials, and cause system failures. For instance, in outdoor electrical installations, continuous corrosion may lead to loose connections, arcing, and eventual equipment malfunction, posing serious safety risks.
Prevention Methods
Use of Anti-Corrosion Compounds
Applying conductive anti-oxidant pastes or gels to aluminum wires before making connections can help prevent galvanic corrosion. These compounds seal the metal interface and reduce moisture ingress, neutralizing electrochemical reactivity.
Barrier Methods
Using bi-metal connectors with zinc-plated or tin-coated interfaces can separate copper and aluminum, effectively breaking the galvanic pathway. Protective coatings, like heat-shrink tubing or epoxy resins, around joints can block access to electrolytes, significantly reducing corrosion risks.
Isolation Techniques
Employing dielectric insulators, such as non-conductive spacers or washers, between the metals can prevent direct metal-to-metal contact, thus minimizing the risk of galvanic corrosion. Installing electrical connections in dry, climate-controlled enclosures can help reduce exposure to moisture and electrolytes, thereby mitigating corrosion.
Understanding the mechanisms of galvanic corrosion and implementing effective prevention strategies are essential for maintaining the integrity of copper-aluminum connections. Regular inspections and adherence to industry standards ensure long-term performance and safety.
Thermal Expansion Mismatch
Thermal expansion mismatch occurs when different materials expand at different rates in response to temperature changes. This is particularly significant in electrical connections involving copper and aluminum wires, which have differing coefficients of thermal expansion: approximately 23 × 10⁻⁶/°C for aluminum and 17 × 10⁻⁶/°C for copper. The higher coefficient of thermal expansion in aluminum means it will expand and contract more than copper when subjected to the same temperature changes. This discrepancy can lead to several issues in electrical connections.
Impact on Electrical Connections
Connection Loosening
Repeated heating and cooling cause aluminum to expand and contract more than copper, which can loosen the connections over time and increase contact resistance. Higher resistance at the connection point results in localized heating, which can exacerbate the problem and potentially cause electrical arcing.
Overhead Line Sagging
In overhead electrical lines, the higher thermal expansion of aluminum can cause the lines to sag more than those made of copper. This increased sagging can lead to safety hazards such as short circuits, particularly in environments where the lines may come into contact with vegetation or other structures.
Corrosion and Oxidation
Loose connections due to thermal expansion mismatch can expose aluminum to air and moisture, speeding up its oxidation. Since copper is less prone to corrosion, the presence of aluminum oxide at the connection point can further degrade the electrical contact, increasing the likelihood of overheating and failure.
Mitigation Strategies
Material Transition Techniques
- CO/ALR-Rated Devices: Using devices specifically rated for copper-aluminum connections can mitigate the effects of thermal expansion mismatch. These devices are designed to handle the differential expansion rates effectively.
- Pigtailing: This involves connecting aluminum wires to short lengths of copper wire using UL-listed connectors. The copper pigtail can then be connected to the terminal, reducing the direct interaction between copper and aluminum.
Installation Best Practices
- Torque Control: Ensuring that terminals are tightened to the manufacturer’s specifications is crucial. Proper torque helps maintain a stable connection, compensating for the natural “creep” of aluminum under pressure.
- Anti-Oxidant Compounds: Applying anti-oxidant pastes to aluminum wires before making connections can help prevent oxidation and maintain a better electrical contact over time.
Design Adaptations
Allowing some slack in aluminum wiring can accommodate its greater thermal movement, which is particularly useful in installations where temperature changes are common. Additionally, avoiding mixed-metal terminations and using terminals and connectors explicitly rated for aluminum reduces the risk of thermal expansion mismatch and improves long-term reliability.
Material Properties Comparison
Conductivity and Electrical Properties
Copper is renowned for its superior electrical conductivity, making it the preferred choice for high-performance applications where efficiency is critical. Copper has about 97% of the maximum possible electrical conductivity, while aluminum has about 61%. This significant difference means that aluminum wires must be larger in diameter to carry the same current as copper, impacting installation space and design.
Thermal Expansion and Resistivity
Copper and aluminum have different coefficients of thermal expansion, with aluminum expanding more than copper when exposed to temperature changes. This difference can cause connections to loosen over time, increasing resistance and the risk of overheating. Copper’s resistivity is lower than aluminum, contributing to its efficiency in electrical applications. However, aluminum’s lighter weight can be advantageous where space and weight are considerations, despite the need for larger wire sizes.
Mechanical Properties: Strength and Durability
Copper is significantly stronger than aluminum, with about 40% more tensile strength. This strength makes copper less prone to breakage and ideal for environments that require robust wiring solutions. Additionally, copper has better creep resistance, meaning it maintains its shape under stress and fluctuating loads better than aluminum. Copper’s flexibility and ductility allow it to be bent and twisted without breaking, which is crucial for installations needing reliable and durable connections.
Weight and Cost Considerations
Aluminum is much lighter than copper, weighing only about 30% as much for the same volume, which is beneficial in industries like aerospace and automotive where weight reduction is paramount. On the cost front, aluminum is generally more economical than copper, providing a cost-effective solution for large-scale electrical installations. However, the need for larger cross-sectional areas to match copper’s conductivity can offset some of these savings in terms of space and material usage.
Risks and Solutions in Electrical Connections
Aluminum can oxidize easily, so connections need anti-oxidation compounds to prevent corrosion. Mixing copper and aluminum requires special connectors, such as Cu-Al connectors, to ensure safe and stable connections. Proper installation practices, including the use of devices rated for aluminum wiring, are crucial to mitigate risks and ensure longevity and safety in electrical systems.
Understanding these material properties is essential for selecting the appropriate wire type based on application needs, considering factors such as conductivity, mechanical strength, weight, and cost to optimize electrical system performance.
Safe Connection Methods
Cu-Al connectors are designed to safely connect copper and aluminum wires by preventing direct contact between the two metals. These connectors are crucial in avoiding galvanic corrosion, ensuring reliable electrical connections, especially in retrofitting older aluminum wiring systems.
Installation Steps
To install Cu-Al connectors effectively:
- Preparation and Connector Selection: Begin by ensuring that both copper and aluminum wires are clean and free from oxidation. Trim any damaged sections and strip the insulation to expose fresh wire. Then, select the appropriate Cu-Al connector based on wire gauge and application needs.
- Wire Insertion: Carefully insert the copper and aluminum wires into their respective ports within the connector.
- Secure Connection: Tighten the connector screws according to the manufacturer’s specifications to establish a firm and stable connection.
Application of Anti-Oxidation Compound
Anti-oxidation compounds like Noalox prevent aluminum wires from oxidizing. These compounds form a protective barrier that reduces the formation of non-conductive oxide layers.
Usage Guidelines
When applying anti-oxidation compounds:
- Application: Generously apply the compound to the exposed aluminum wire before inserting it into the connector.
- Distribution: Ensure the compound is evenly spread to cover all exposed surfaces, creating an effective protective layer.
- Connector Installation: Proceed with the connector installation, ensuring the compound does not interfere with the mechanical connection.
Compliance with NEC/IRC Standards
Compliance with NEC and IRC standards is essential for safe electrical installations. Specific codes govern the use of connectors and anti-oxidation compounds for copper and aluminum wire connections.
Specific Codes
- NEC 3406.8: Specifies requirements for safe connections between copper and aluminum wires, including using listed connectors and anti-oxidation compounds.
- UL 486C: Details the testing and certification standards for connectors used in mixed-metal connections.
Inspection Requirements
Regular inspections are vital to maintaining the integrity of copper-aluminum connections. Inspections should check for signs of wear, oxidation, and loose connections to ensure ongoing safety and reliability.
Installation Methods
Wire nuts and pigtailing are effective methods for connecting copper and aluminum wires. Wire nuts should be rated for mixed-metal connections, while pigtailing involves connecting aluminum wires to short lengths of copper wire using specialized connectors. These methods reduce direct contact between the metals and minimize oxidation risks, contributing to safer and more reliable electrical systems.
Electrical Safety Considerations
Connecting copper and aluminum wires directly can lead to serious safety issues, which need to be understood and addressed to prevent electrical hazards.
One primary concern with direct copper-aluminum connections is the potential for overheating, which can lead to electrical fires. Aluminum expands more than copper, which can loosen connections over time, leading to increased resistance and heat. Loose connections may result in arcing, a dangerous condition where electricity jumps across gaps, potentially igniting surrounding materials.
Direct contact between copper and aluminum can cause corrosion due to their different metal reactions. This corrosion increases resistance at the connection point, exacerbating the risk of overheating. Additionally, aluminum wires are more prone to oxidation compared to copper. Oxidation forms a resistive layer on the wire surface, hindering electrical conductivity and increasing the risk of overheating. This resistive layer can cause connections to fail, leading to arcing and potential fire hazards.
Using specialized connectors designed for copper-aluminum wire connections is essential to prevent direct metal contact and mitigate the risks of corrosion. These connectors create a secure barrier between the metals, reducing corrosion and enhancing the safety of the connection.
Applying anti-oxidation compounds to aluminum wires before making connections helps prevent the formation of resistive oxide layers. These compounds provide a protective barrier that maintains conductivity and minimizes the risk of overheating due to oxidation.
Following safety standards like the NEC and IRC is essential for safe electrical work. These standards specify the use of approved connectors and materials to maintain safe and reliable connections between copper and aluminum wires.
Hiring licensed electricians for installation and regular inspection of copper-aluminum connections is vital to ensure safety and compliance with electrical standards. Professionals can identify potential issues such as loose connections or signs of corrosion and take corrective measures to prevent hazards.
Managing electrical loads effectively can prevent excessive strain on copper-aluminum connections, reducing the risk of overheating. Avoiding overloading outlets and circuits helps maintain stable connections and minimizes the potential for electrical fires.
By understanding the risks associated with copper-aluminum wire connections and implementing appropriate safety measures, it is possible to ensure the longevity and safety of electrical systems.
Frequently Asked Questions
Below are answers to some frequently asked questions:
What happens if you connect copper and aluminum wires directly?
Directly connecting copper and aluminum wires can lead to several risks, primarily due to galvanic corrosion and thermal expansion differences. When these dissimilar metals come into contact, they can form an electrochemical cell in the presence of moisture, causing the aluminum to oxidize faster. This results in aluminum oxide buildup, which increases electrical resistance, leading to overheating and potential fire hazards. Additionally, aluminum expands and contracts more significantly than copper with temperature changes, known as “cold creep,” which can loosen connections over time, further increasing resistance and arcing risks. To mitigate these issues, it’s crucial to use approved connectors that prevent direct metal contact, such as Al/Cu-rated wire nuts, and apply anti-oxidant paste to seal connections from moisture and air. Implementing these measures not only enhances safety but also ensures compliance with electrical codes.
How to safely connect copper and aluminum wires?
To safely connect copper and aluminum wires, follow approved methods and precautions. Use UL-listed connectors like AlumiConn or COPALUM crimps to prevent direct metal contact and ensure secure connections. AlumiConn connectors have separate ports for each wire, while COPALUM crimps require professional installation to create a cold-weld bond, eliminating oxidation risks.
Another method is pigtail splicing, where a short copper wire is attached to the aluminum wire using UL-listed connectors. The copper end is then connected to devices, reducing stress on the aluminum wire.
Applying antioxidant compounds such as Noalox to aluminum wire ends before connecting can inhibit oxidation, reducing resistance and overheating risks.
Direct contact between copper and aluminum can cause galvanic corrosion, especially in humid environments. Therefore, always use connectors with separate ports or dielectric grease to block moisture.
Additionally, aluminum expands and contracts more than copper, which can loosen connections over time. Ensure connectors are rated for aluminum and are torqued to manufacturer specifications to mitigate this issue.
Compliance with NEC standards is crucial, and methods like COPALUM crimping require certified electricians. Regular inspections for discoloration, warmth, or flickering lights can indicate loose connections. Following these guidelines reduces hazards and ensures safe connections.
Why does aluminum oxidize faster than copper?
Aluminum oxidizes faster than copper primarily due to its higher reactivity and electronegativity. Aluminum is more prone to oxidation because it readily forms a hard, electrically insulating oxide layer that quickly re-establishes itself if disturbed. This aluminum oxide layer can increase electrical contact resistance and complicate wire terminations. In contrast, copper forms a softer, electrically conductive oxide layer that can be easily disrupted under mechanical pressure, maintaining better electrical contact.
Additionally, aluminum is more susceptible to galvanic corrosion when in contact with more cathodic metals like copper, especially in moist environments. This susceptibility further contributes to its rapid oxidation compared to copper. Understanding these differences is essential for managing risks associated with aluminum wire connections and ensuring reliable electrical systems, as explored throughout the article.
What are the NEC/IRC standards for wire connections?
The NEC (National Electrical Code) and IRC (International Residential Code) set specific standards for safely connecting copper and aluminum wires. These standards aim to mitigate risks such as galvanic corrosion and thermal expansion mismatch. Key requirements include using connectors explicitly rated for copper-to-aluminum connections, often marked “CO/ALR,” which undergo rigorous testing to ensure compatibility. Push-in terminals are restricted to solid copper or copper-clad aluminum, not pure aluminum. Conductors must be tightened to specified torque values to prevent loosening and arcing. Installation instructions for aluminum wiring emphasize proper technique, discouraging pre-twisting unless connectors are rated for it. Compliance with these standards ensures safe and effective electrical connections, reducing risks of overheating and electrical fires. Regular inspections should verify the use of appropriate connectors and adherence to workmanship guidelines, as discussed earlier. These practices are crucial for maintaining electrical safety in residential settings.
Can I use regular wire nuts for copper and aluminum connections?
Regular wire nuts are not suitable for connecting copper and aluminum wires. These standard wire nuts are designed primarily for copper-to-copper connections and do not address the specific issues that arise with aluminum, such as oxidation and thermal expansion. Using regular wire nuts for copper and aluminum connections can lead to increased resistance, overheating, and potentially dangerous conditions like electrical fires. Instead, specialized connectors, such as the IDEAL Twister Al/Cu wire connectors, are recommended. These connectors are specifically designed for aluminum-to-copper connections and include features like antioxidant compounds to prevent oxidation and ensure a secure, safe connection.
How can thermal expansion affect wire connections?
Thermal expansion can significantly impact wire connections, particularly when using materials like copper and aluminum. As these metals expand and contract with temperature changes, mechanical stress is induced at connection points. This stress can lead to loosening and degradation of the connections over time. Copper and aluminum have different thermal expansion rates, with aluminum expanding more for a given temperature change. This mismatch can exacerbate the stress at junctions where the two metals meet, increasing the risk of connection failure. To mitigate these effects, special connectors designed to accommodate thermal expansion differences should be used, and connections should be periodically inspected and maintained.
