In the realm of materials science, the understated brilliance of zinc often goes unnoticed, yet its role in modern technology is indispensable. How does this humble element transform into a powerhouse of versatility through the intriguing dance of metallic bonding? This comprehensive guide unravels the complexities of zinc’s metallic bonding, exploring how electron delocalization and ionic radius shape its unique properties. Delve into a technical deep-dive to understand why zinc stands apart from metals like sodium and magnesium, and discover its implications across industries from construction to electronics. As we peel back the layers of zinc’s bonding mechanisms, prepare to uncover the secrets behind its conductivity, malleability, and more. Could the key to future innovations lie within zinc’s metallic embrace? Let’s explore.
Introduction to Metallic Bonding
Metallic bonding is a type of chemical bonding found in metals, where electrons are shared collectively among a lattice of metal atoms. This unique interaction results in properties that distinguish metals from other types of substances.
The electron sea model is central to understanding metallic bonding. In this model, metal atoms release their valence electrons, allowing them to move freely across the entire metal structure. These free-moving electrons form a sea of negative charge that surrounds the metal ions, holding the structure together. This electron mobility is responsible for many of the distinctive properties of metals, such as conductivity and malleability.
Metallic bonding is vital in materials science and chemistry, influencing the behavior and properties of metals significantly. The ability of electrons to move freely within the metallic lattice contributes to several key characteristics:
- Electrical Conductivity: Metals are excellent conductors of electricity due to the presence of free electrons that can carry charge throughout the structure. This is why metals like copper are widely used in electrical wiring.
- Thermal Conductivity: These free electrons also efficiently transfer heat, which makes metals great conductors of thermal energy—ideal for cookware and heat exchangers.
- Malleability and Ductility: The non-directional nature of metallic bonds allows metal ions to slide past each other without breaking the bond, enabling metals to be hammered into thin sheets (malleability) or stretched into wires (ductility).
Several factors influence the strength of metallic bonds. The number of delocalized electrons and the ionic radius play key roles in determining bond strength. Metals with more delocalized electrons generally exhibit stronger metallic bonds. Transition metals, with their partially filled d-orbitals, often have higher bond strengths compared to alkali metals. Additionally, smaller ionic radii lead to higher charge density and stronger electrostatic interactions between the ions and the electron sea. This explains why metals with smaller atoms, like zinc, may exhibit stronger metallic bonds.
Understanding these principles is fundamental for applications in metallurgy, electronics, and the design of materials with specific properties tailored to industrial needs. As technology advances, the study of metallic bonding continues to evolve, incorporating quantum mechanics to better predict and manipulate metal behavior in complex systems.
Mechanisms of Metallic Bonding in Zinc
Overview of Zinc as a Material
Zinc, represented by the chemical symbol Zn, is a versatile metal known for its unique properties, including its ability to form alloys and its extensive use across various industries. These properties are fundamentally influenced by zinc’s atomic structure and bonding characteristics, particularly through the mechanisms of metallic bonding.
Metallic Bonding in Zinc
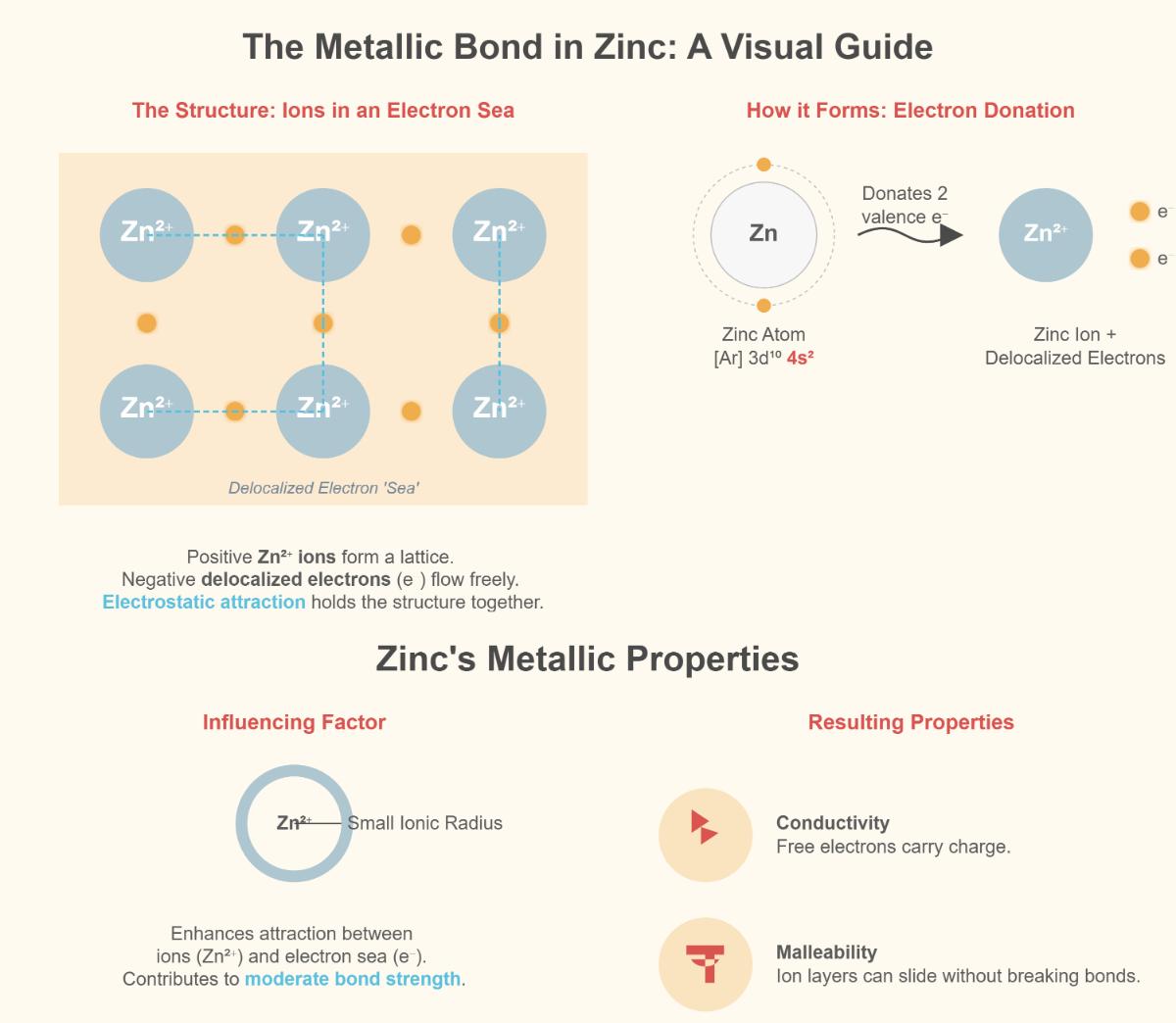
Metallic bonding in zinc involves a lattice of positively charged zinc ions enveloped by a sea of delocalized electrons. This electron cloud facilitates strong electrostatic attractions between the ions, providing stability to the metallic structure. Unlike ionic or covalent bonds, metallic bonds do not involve the sharing or transferring of electrons between specific atoms. Instead, the electrons are free to move throughout the metal, contributing to zinc’s conductivity and malleability.
Role of Electron Delocalization
The free movement of electrons allows zinc to conduct electricity efficiently, as they can carry charge throughout the metal. This also enables zinc to transfer heat rapidly, contributing to its thermal conductivity. The delocalization of electrons is a key factor in zinc’s ability to efficiently conduct both electricity and heat.
Influence of Ionic Radius
Zinc has a relatively small ionic radius, which results in a higher charge density. This high density strengthens the electrostatic attraction between the ions and the electron cloud, leading to stronger metallic bonds. The compact arrangement of zinc ions allows layers within the crystal structure to slide over one another, enhancing its ductility and malleability.
Crystal Structure and Bonding
Zinc crystallizes in a hexagonal close-packed (hcp) structure, where each zinc ion is surrounded by twelve nearest neighbors. This efficient packing contributes to zinc’s mechanical properties. The non-directional nature of metallic bonds in zinc’s crystal structure permits ions to slide over each other without breaking the bonds, facilitating plastic deformation under stress.
Hybridization and Covalent Influence
While metallic bonding is predominant in zinc, there are also minor covalent interactions within its crystal structure. This hybridization involves the overlap of d and p orbitals, adding complexity to zinc’s bonding characteristics. These covalent interactions, though less significant than metallic bonds, can influence zinc’s reactivity and interaction with other elements, impacting its applications in various chemical processes.
Implications for Material Properties
The mechanisms of metallic bonding in zinc are crucial for its chemical and physical properties. The free movement of electrons contributes to zinc’s ability to form alloys with other metals, enhancing properties like corrosion resistance and mechanical strength. Understanding these bonding mechanisms allows scientists and engineers to manipulate zinc’s characteristics for specific industrial applications, from galvanization to battery technology.
Properties of Zinc Due to Metallic Bonding
Zinc’s chemical properties are greatly influenced by its metallic bonding. This electron delocalization allows zinc to easily combine with other metals, forming alloys like brass that are both strong and resistant to corrosion. The metallic bonding also contributes to zinc’s effectiveness as a galvanizing agent, providing a protective barrier against oxidation and rust in steel products.
Thanks to its ability to conduct electricity, zinc is an essential component in dry cell batteries and galvanic coatings. The presence of delocalized electrons in zinc’s metallic structure results in moderate electrical conductivity, which is useful for various electrical applications.
While zinc is typically hard and brittle at room temperature, it becomes more workable when heated between 100°C and 150°C. This temperature-dependent malleability is due to the ability of zinc ions to slide past each other without disrupting the metallic bonds, allowing zinc to be shaped and formed for various industrial uses, including die-casting and sheet metal production.
Zinc’s metallic bonding also contributes to its thermal conductivity. The free electrons within zinc’s structure enable efficient heat transfer, making zinc useful in applications where heat dissipation is crucial. This property is exploited in manufacturing processes where zinc is used as a component in heat exchangers and thermal management systems.
The strength of metallic bonding in zinc is reflected in its melting and boiling points. Zinc melts at 419.5°C and boils at 907°C, which are relatively high compared to metals with weaker metallic bonds. These thermal properties are advantageous in high-temperature applications where zinc must maintain structural integrity, such as in casting and alloy production.
These properties make zinc a valuable choice in industries like automotive and construction, where its durability and resistance to corrosion are crucial.
Comparison with Sodium and Magnesium
Metallic Bonding in Sodium
Sodium (Na) has relatively weak metallic bonding because of its simple electron configuration. The sodium atom contains a single valence electron in the 3s orbital ([Ne]3s¹), which contributes to a less dense electron sea compared to metals with more delocalized electrons. In sodium’s metallic lattice, Na⁺ ions are attracted to these delocalized electrons, but the positive charge is only +1 and the ionic radius is relatively large, resulting in weaker electrostatic forces.
Properties of Sodium Due to Metallic Bonding
Sodium is soft and highly malleable, easily cut with a knife, due to its weak metallic bonding and low electron density. Its melting point is approximately 98°C, relatively low for a metal, as the weak bonds require less energy to break. Despite moderate conductivity from its single valence electron, sodium is not as efficient in conducting electricity as metals with stronger metallic bonds.
Metallic Bonding in Magnesium
Magnesium (Mg) exhibits strong metallic bonding compared to sodium. Mg²⁺ ions, with a +2 charge and smaller size than Na⁺ ions, create stronger metallic bonds. Magnesium atoms have two valence electrons in the 3s orbital ([Ne]3s²), resulting in a higher density of delocalized electrons and enhanced electrostatic attractions within the metallic structure.
Properties of Magnesium Due to Metallic Bonding
Magnesium is renowned for its high strength-to-weight ratio, making it ideal for lightweight applications requiring durability. With a melting point of approximately 650°C, magnesium’s metallic bonds are significantly stronger, requiring more energy to break. The increased electron density also enhances both electrical and thermal conductivity, making magnesium suitable for electronic and heat dissipation applications.
Comparative Analysis with Zinc
Electron Configuration and Delocalization
- Sodium: [Ne]3s¹ (one delocalized electron)
- Magnesium: [Ne]3s² (two delocalized electrons)
- Zinc: [Ar]3d¹⁰4s² (two delocalized electrons, with localized d-electrons)
Zinc’s fully filled d-subshell (3d¹⁰) influences its metallic bonding. Despite having two delocalized electrons in the 4s orbital, the filled d-electrons limit the efficiency of electron delocalization compared to magnesium.
Ionic Charge and Radius
- Sodium: +1 charge, larger ionic radius
- Magnesium: +2 charge, smaller ionic radius
- Zinc: +2 charge, small ionic radius
Both zinc and magnesium have a +2 charge on their ions, but zinc’s ionic radius is slightly larger than magnesium’s due to the filled d-subshell. This results in intermediate bond strength, stronger than sodium but slightly weaker than magnesium.
Physical Properties
- Melting Point:
- Sodium: ~98°C
- Magnesium: ~650°C
- Zinc: ~420°C
Zinc’s melting point reflects its intermediate bond strength. Magnesium’s higher melting point is due to the stronger metallic bonding facilitated by efficient electron delocalization.
- Bond Strength:
- Sodium: Weakest
- Magnesium: Strongest
- Zinc: Moderate
Zinc’s bond strength is balanced between the weak bonds of sodium and the strong bonds of magnesium, influenced by its electron configuration and ionic characteristics.
Practical Implications in Industry
Sodium has limited structural applications due to its weak bonds and is mainly used in chemical reactions. Magnesium is preferred for high-strength, lightweight components in aerospace and automotive industries. Zinc is ideal for galvanization, batteries, and applications requiring moderate strength and corrosion resistance. Understanding the comparative metallic bonding in sodium, magnesium, and zinc provides insights into their unique properties and industrial applications. This knowledge helps in selecting appropriate metals for specific uses based on their bonding characteristics and resulting physical properties.
Real-World Applications of Zinc
Galvanization and Corrosion Resistance
Zinc plays a crucial role in galvanization, acting as a protective layer for steel or iron. This application is essential in preventing corrosion and significantly extending the lifespan of steel structures and components. The metallic bonding in zinc allows it to form a durable and adherent layer that shields the underlying metal from environmental factors, such as moisture and oxygen, which can lead to rust and deterioration. Galvanized steel is prevalent in construction, infrastructure, and automotive industries due to its enhanced durability and resistance to corrosive elements.
Die Casting and Alloy Formation
Zinc’s low melting point makes it ideal for die casting, a process used to create intricate, high-precision components in the automotive and electronics industries. Zinc alloys, such as Zamak, are frequently used in die casting, offering excellent strength and durability. Additionally, zinc is a key component in the formation of brass, an alloy renowned for its corrosion resistance and aesthetic appeal, commonly used in plumbing fixtures and musical instruments.
Chemical and Pharmaceutical Industry Uses
Zinc is essential in the chemical industry, where it is used to produce various compounds, including zinc oxide and zinc sulfate. These compounds play crucial roles in different sectors; zinc oxide is widely used in rubber manufacturing, cosmetics, and pharmaceuticals, particularly in sunscreen formulations for its UV protective properties. Zinc sulfate is used in agriculture to improve plant growth and animal health.
Electroplating and Surface Enhancement
In electroplating, zinc creates a protective layer on metal surfaces, boosting corrosion resistance and enhancing appearance. Zinc-nickel electroplating, in particular, offers superior durability and is compatible with rubber adhesion, making it ideal for automotive and industrial uses. This process improves the longevity and performance of coated products, providing a robust barrier against environmental degradation.
Influence on Electrical and Mechanical Properties
Zinc’s metallic bonding contributes to its moderate electrical conductivity, making it a valuable component in dry cell batteries and galvanic coatings. Zinc’s ductility and malleability enable it to be shaped into products like sheets and wires without losing strength. These properties are harnessed in manufacturing processes where zinc’s ability to efficiently conduct electricity and heat is exploited, particularly in thermal management systems and heat exchangers.
Zinc’s versatility in these applications underscores its importance in modern industrial practices, where its unique properties derived from metallic bonding are continuously leveraged to innovate and improve product performance across multiple sectors.
Interactive Models and Diagrams
Interactive models are essential for visualizing electron delocalization in metallic bonding, especially in metals like zinc. These models simulate the dynamic environment where electrons move freely around a lattice of positively charged zinc ions, allowing learners to observe the “sea of electrons” and understand its role in properties such as conductivity and malleability.
Diagrams play a critical role in depicting the structural arrangement of zinc atoms in a metallic bond. A typical diagram would feature a dense, regular pattern of zinc atoms, with zinc ions represented as spheres, surrounded by a cloud of delocalized electrons. This visual representation helps in grasping how the metallic bonds are formed and maintained, as well as how the ions can slide past each other without breaking the bond, illustrating zinc’s ductility.
Various interactive tools and resources provide hands-on experiences with metallic bonding concepts. Virtual labs and 3D simulations let users manipulate atomic structures and see how changing variables like temperature or electron density affects zinc’s properties. These tools often include quizzes and guided activities that reinforce learning by challenging users to apply their knowledge in simulated scenarios.
Several educational software platforms and apps are designed to facilitate a deeper understanding of metallic bonding. These resources offer features such as zoomable 3D models, adjustable parameters for ionic size and charge, and real-time feedback on user interactions. By engaging with these tools, students and professionals alike can explore the nuances of metallic bonding in zinc and compare it to other metals, enhancing both theoretical knowledge and practical insight.
Interactive models and diagrams bridge the gap between theory and practice, making complex phenomena easier to grasp and fostering a deeper understanding of metallic bonding and zinc’s applications. By providing a more engaging and immersive learning experience, these tools help enhance comprehension of metallic bonding and its implications for zinc’s properties and applications.
Frequently Asked Questions
Below are answers to some frequently asked questions:
How does metallic bonding occur in zinc?
Metallic bonding in zinc occurs through a lattice structure composed of positively charged zinc ions (Zn²⁺) surrounded by a “sea” of delocalized valence electrons. Zinc, with an electron configuration of [Ar]3d¹⁰4s², donates its two 4s electrons to this electron sea, allowing these electrons to move freely throughout the metal lattice. This electron delocalization leads to electrostatic attractions between the positively charged zinc ions and the negatively charged electrons, creating the metallic bond. Zinc’s relatively small ionic radius enhances the attraction between the zinc ions and the delocalized electrons, contributing to its moderate bond strength and characteristic properties such as conductivity and malleability.
What are the unique properties of zinc due to its metallic bonding?
Zinc’s unique properties arise from its metallic bonding, where zinc atoms contribute two valence electrons to a delocalized “sea of electrons” within the metal lattice. This electron delocalization results in strong electrostatic attractions between positively charged zinc ions and free electrons, contributing to zinc’s notable properties. Zinc’s melting point of 419.5 °C and boiling point of 907 °C, while lower compared to some metals, reflect the strength of its metallic bonds. Zinc is a fair conductor of electricity, thanks to the free electrons facilitating charge transfer. Mechanically, zinc is hard and brittle at room temperature but becomes malleable between 100 °C and 150 °C, reverting to brittleness above 210 °C. Additionally, zinc’s tendency to form stable complexes and alloys, such as brass with copper, underscores its versatility and industrial significance. These properties make zinc a valuable material in various applications, including construction and electronics.
How does the metallic bonding in zinc compare to that in other metals?
Zinc’s metallic bonding is characterized by its two valence electrons and a relatively small ionic radius, contributing to strong electrostatic attractions between zinc ions and delocalized electrons. This results in properties such as high melting and boiling points, mechanical resilience, and good electrical conductivity. When compared to sodium, an alkali metal with only one valence electron, zinc exhibits stronger metallic bonding due to greater electron density and smaller ionic radius, leading to more robust mechanical and thermal properties.
In contrast to magnesium, an alkaline earth metal with similar two valence electrons, zinc has a partially filled 3d subshell that slightly shields its valence electrons, making its metallic bonding somewhat weaker than magnesium’s. This results in magnesium having higher melting and boiling points than zinc. Zinc’s balanced properties make it ideal for applications requiring moderate mechanical strength and corrosion resistance, such as galvanization and alloy production, distinguishing it from the weaker bonding in sodium and the stronger bonding in magnesium.
What are some real-world applications of zinc due to its metallic bonding?
Zinc’s metallic bonding properties enable its wide range of industrial applications. One key application is galvanization, where zinc is used to coat steel or iron, providing corrosion resistance through the formation of a protective zinc oxide layer. Zinc’s low melting point and ability to form complex shapes make it ideal for die casting, particularly in automotive and electronics industries. Additionally, zinc is crucial in the creation of alloys like brass and Zamak, which are valued for their strength, durability, and corrosion resistance. In the chemical industry, zinc’s reactivity leads to the production of compounds like zinc oxide and zinc sulfate, used in rubber manufacturing, cosmetics, and agriculture. Lastly, zinc-nickel electroplating is employed for its corrosion resistance and compatibility with rubber, enhancing the durability of various products. These applications demonstrate the versatility and importance of zinc due to its metallic bonding characteristics.
Can you provide visual models to help understand metallic bonding in zinc?
Yes, visual models can significantly aid in understanding metallic bonding in zinc. The electron sea model is a common depiction, where zinc atoms are represented as a lattice of positively charged Zn²⁺ ions immersed in a “sea” of delocalized electrons. This model helps visualize how these free-moving electrons provide metallic properties like electrical conductivity and malleability.
Additionally, vector illustrations can show the hexagonal close-packed (HCP) structure of zinc, highlighting the arrangement of Zn²⁺ ions and the electron cloud. Comparative diagrams contrasting zinc with sodium and magnesium can further illustrate differences in bonding strength and electron distribution.
3D representations and animations can also be useful. For example, a dynamic model showing how electrons redistribute when zinc is subjected to mechanical stress can explain its ductility. These visual tools, available on various educational and scientific platforms, provide a clear and interactive way to grasp the concept of metallic bonding in zinc.
Why is zinc preferred in certain industrial applications over other metals?
Zinc is preferred in certain industrial applications over other metals due to several unique properties stemming from its metallic bonding. These properties include excellent corrosion resistance, which is crucial for protecting steel through galvanizing and coatings, particularly in harsh environmental conditions. Zinc’s ability to provide cathodic protection further enhances its utility in safeguarding steel structures.
Additionally, zinc’s malleability and moderate melting point make it highly suitable for manufacturing processes, as it can be easily shaped and formed, reducing production costs and energy consumption. Zinc is also highly recyclable, maintaining its properties through repeated cycles, contributing to sustainability and a lower environmental impact.
Furthermore, zinc’s cost-effectiveness and energy efficiency, due to its lower production energy requirements compared to other metals, make it an attractive choice. Its electrical conductivity is valuable in applications such as battery production. These combined factors make zinc a versatile and beneficial material in various industrial applications.