When it comes to protecting metals from corrosion, the choice between zinc plating and passivation can be crucial, yet perplexing. What sets these two methods apart, and how do you decide which is best for your needs? Zinc plating and passivation both offer unique benefits and are suited for different applications, but understanding their primary differences is key. In this article, we’ll delve into the specifics of each process, compare their effectiveness in corrosion resistance, and examine the environmental considerations that may influence your decision. So, which one holds the edge—zinc plating or passivation? Read on to find out.
Understanding Zinc Plating and Passivation
Zinc plating is a popular metal finishing technique that involves applying a protective zinc layer to metal surfaces, primarily to prevent corrosion and improve appearance. The process starts with thoroughly cleaning the substrate to ensure a smooth surface for plating. During electroplating, the metal object is immersed in a zinc ionic solution, and an electrical current is applied to deposit zinc ions onto the substrate’s surface. Zinc plating uses different types of solutions, such as acid and alkaline, which affect the speed and thickness of the coating. Acid zinc solutions are suitable for high-volume production due to their faster deposition rates, while alkaline solutions can achieve greater coating thickness for enhanced protection.
Passivation is a chemical process that treats metal surfaces to form a stable oxide layer, enhancing corrosion resistance after plating. This treatment begins with cleaning the metal to remove impurities, followed by applying a passivation solution that reacts with the surface to create the protective oxide layer. The process concludes with rinsing and drying to prevent further reactions, thereby enhancing the surface’s durability against environmental factors.
| Feature | Zinc Plating | Passivation |
|---|---|---|
| Process | Electrodeposition of zinc onto metal | Chemical treatment to form oxide layer |
| Purpose | Corrosion protection, aesthetic enhancement | Enhance corrosion resistance, stabilize surface |
| Thickness | Thickness varies based on the solution and method used | No additional material deposited |
| Durability | Provides a durable protective layer | Enhances existing surface durability |
Both zinc plating and passivation are vital for boosting metal durability and corrosion resistance, with the choice depending on specific needs like protection level and aesthetics. Recent advancements in these techniques focus on increasing efficiency and sustainability, aligning with evolving environmental standards and industrial demands.
Comparing Zinc Plating and Passivation Processes
Process Overview
When comparing zinc plating and passivation, understanding each process’s intricacies is essential. Zinc Plating involves electrochemical deposition, where a zinc layer is applied to metal surfaces, such as steel. This process forms a sacrificial protective layer that acts as a physical barrier, corroding preferentially to shield the underlying metal. The deposition thickness can vary, typically measured in microns, which influences the level of protection and durability.
Passivation is a chemical treatment applied to zinc-coated metals to enhance their corrosion resistance. It uses chromate or trivalent solutions to form a thin conversion layer, known as zinc chromate. This enhances the zinc layer by preventing the formation of white rust. Unlike zinc plating, passivation does not add thickness but rather modifies the existing surface to stabilize it against environmental influences.
Key Differences
Layer Thickness and Corrosion Resistance
- Zinc Plating: Produces a thick zinc coating that offers substantial protection, particularly in harsh environments. It provides high corrosion resistance due to its sacrificial nature, where zinc corrodes first to protect the base metal.
- Passivation: Results in an ultra-thin chemical conversion layer that enhances the existing zinc coating without adding significant thickness. It improves corrosion resistance by preventing zinc oxidation and extending the lifespan of the zinc layer.
Durability and Cost Considerations
- Zinc Plating: Known for its superior durability, especially suitable for outdoor or marine applications. Generally incurs higher costs due to the complexity and materials involved in the plating process.
- Passivation: While less durable than plating, it is ideal for precision parts where dimensional stability is crucial. It offers a lower cost alternative as a post-treatment step, making it more economical for applications where plating is already present.
Aesthetic Appeal
- Zinc Plating: Provides a bright, metallic finish that is visually appealing for decorative purposes.
- Passivation: Offers various color options, including yellow and black finishes, which can be tailored to specific aesthetic requirements.
Applications
Zinc Plating is predominantly used for components exposed to outdoor conditions, such as marine hardware and fasteners, where robust protection is paramount. Passivation finds its niche in electronics and precision parts, where maintaining dimensional integrity and preventing hydrogen embrittlement—a condition that makes metals brittle due to hydrogen absorption—are critical.
Technical Considerations
Hydrogen Embrittlement is a condition that makes metals brittle due to hydrogen absorption. Passivation reduces the risk associated with this compared to zinc plating, making it preferable for delicate applications. Environmental Compliance: Hexavalent chromium passivation is effective but faces regulatory restrictions; trivalent alternatives comply with standards like RoHS.
Recent Trends
The industry is shifting towards hybrid processes combining zinc plating and passivation to optimize both corrosion resistance and cost efficiency. Zinc plating remains favored for environments demanding extreme durability, while passivation gains traction in electronics due to its precision and minimal impact on part dimensions. Regulatory shifts encourage the adoption of trivalent chromium passivation, aligning with global environmental standards.
Corrosion Protection Mechanisms
Corrosion Protection Mechanisms: Zinc Plating
Zinc plating primarily protects metals through sacrificial protection. In this process, called galvanic protection, the zinc corrodes first, protecting the steel from rust. The zinc layer also acts as a physical barrier, shielding the metal from moisture and oxygen, which prevents corrosion.
Self-Healing Properties
Zinc plating exhibits self-healing properties, especially when minor damage occurs. If the zinc layer is scratched or damaged, the exposed steel can still be protected. Zinc reacts with oxygen to form zinc oxide, which then transforms into zinc hydroxide and zinc carbonate, creating a protective layer that continues to shield the steel from further corrosion.
Formation of Protective Compounds
Upon exposure to the atmosphere, zinc reacts with environmental elements to form a series of protective compounds, including zinc oxide, zinc hydroxide, and zinc carbonate. These compounds enhance the corrosion resistance of the zinc layer by providing an additional protective barrier.
Corrosion Protection Mechanisms: Passivation
Passivation, especially effective for stainless steel, uses different mechanisms than zinc plating to enhance corrosion resistance:
Removal of Contaminants
The passivation process starts with the removal of free iron particles and other contaminants from the metal surface. These contaminants can act as initiation sites for corrosion. By eliminating these potential sites, passivation reduces the risk of localized corrosion.
Formation of a Passive Layer
During passivation, the metal is treated with a chemical solution that facilitates the formation of a thin, stable oxide layer on its surface. This passive layer is highly resistant to corrosion and acts as a barrier to further reactions with environmental agents such as oxygen and moisture.
Enhanced Corrosion Resistance
The passive layer from passivation significantly boosts the metal’s resistance to corrosion, extending its lifespan and maintaining its integrity.
Comparative Analysis of Corrosion Protection Mechanisms
| Mechanism | Zinc Plating | Passivation |
|---|---|---|
| Protection Method | Sacrificial coating, barrier effect | Removal of contaminants, formation of passive layer |
| Self-Healing | Offers self-healing through the formation of protective compounds | Generally does not offer self-healing properties |
| Application | Primarily for ferrous metals like steel | Typically for stainless steel |
| Durability | Long-lasting due to sacrificial properties | Highly durable under stable conditions |
| Environmental Impact | Can be more environmentally friendly than some chemical treatments | Involves use of chemicals that may require careful handling |
Choice of Method
- Zinc Plating: Best suited for ferrous metals in harsh environments due to its sacrificial nature and barrier effect.
- Passivation: Ideal for stainless steel, enhancing its natural corrosion resistance by removing contaminants and forming a passive layer.
Regulatory Impact and Environmental Compliance
Both zinc plating and passivation are governed by various regulations to ensure environmental safety and human health. These include the RoHS directive, REACH regulation, and ASTM B633 standard, which collectively dictate acceptable practices and materials in metal finishing processes.
The RoHS directive restricts the use of hazardous substances in electrical and electronic equipment, leading to a shift from hexavalent chromium to trivalent chromium in passivation. Trivalent chromium is less toxic and poses fewer environmental and health risks, making it a preferable alternative despite potential differences in finish appearance. Similarly, REACH ensures that chemical substances used in these processes are managed effectively to mitigate risks. Compliance with the ASTM B633 standard ensures that zinc plating meets industry standards for quality and durability.
Environmental compliance is crucial for both zinc plating and passivation processes. Zinc plating can produce significant waste, including hazardous by-products. Proper disposal is essential to prevent environmental damage. Passivation processes, particularly those using chromates, face scrutiny due to their environmental and health risks. Trivalent chromium-based passivation solutions offer a more environmentally friendly option, aligning with current regulations. These solutions are less toxic and easier to manage, reducing the environmental burden.
Comparative Analysis
| Aspect | Zinc Plating | Zinc Passivation |
|---|---|---|
| Regulatory Compliance | Requires adjustments for RoHS compliance | Generally compliant with RoHS using trivalent chromium |
| Environmental Impact | More resource-intensive, generates significant waste | Less resource-intensive, requires chemical waste management |
| Durability and Protection | Offers thicker, more durable coatings suitable for harsh environments | Provides thinner coatings, enhancing existing protection |
| Waste Management | Requires stringent waste disposal practices | Involves careful handling of chemical solutions |
Trivalent vs Hexavalent Chromium
- Trivalent Chromium:
- Pros: RoHS compliant, less toxic, safer for the environment.
- Cons: May change finish appearance, possibly less effective in some uses.
- Hexavalent Chromium:
- Pros: Highly effective in corrosion resistance, industry-standard.
- Cons: Highly toxic, carcinogenic, restricted by regulations.
Due to regulatory and environmental concerns, chromate-free passivation options are becoming more common. These alternatives aim to provide effective corrosion resistance without using hazardous chromates, aligning with strict environmental standards and improving safety.
Industrial Applications and Recommendations
Understanding how zinc plating and passivation are used in industry is essential for choosing the best method for specific metal finishing needs. Both processes provide unique benefits that align with various industrial requirements.
Zinc plating is extensively applied to automotive parts, such as bolts, nuts, and fasteners, due to its excellent corrosion resistance and aesthetic finish, ensuring longevity and reliability of parts exposed to moisture and road salt. In construction, structural elements and fasteners benefit from zinc plating due to its durability and ability to withstand outdoor conditions, helping prevent rust and maintain structural integrity. In marine settings, zinc plating provides an ideal protective solution for hardware and fasteners, ensuring they resist saltwater corrosion.
Passivation is particularly effective in industries where enhancing the corrosion resistance of existing zinc-coated materials is necessary, and where maintaining dimensional accuracy is crucial. In the aerospace sector, passivation enhances the corrosion resistance of lightweight materials, preventing the formation of white rust and maintaining the structural integrity of components. Passivation is preferred in electronics manufacturing for enhancing corrosion resistance without altering the dimensions of delicate parts, which is essential for ensuring component reliability and performance.
The choice between zinc plating and passivation should be guided by the specific requirements of the application. Zinc plating is suitable when high durability and resistance to extreme environments are priorities, when aesthetic enhancement is desired, or when the application involves exposure to moisture, chemicals, or salt. On the other hand, passivation is ideal when the primary goal is to enhance corrosion resistance of pre-coated materials, maintain dimensional stability, or comply with environmental regulations using less hazardous chemicals. Both methods have their place in industrial applications, and selecting the right one depends on balancing factors such as cost, environmental impact, durability, and specific functional requirements of the finished product.
Frequently Asked Questions
Below are answers to some frequently asked questions:
What’s the primary difference between zinc plating and passivation?
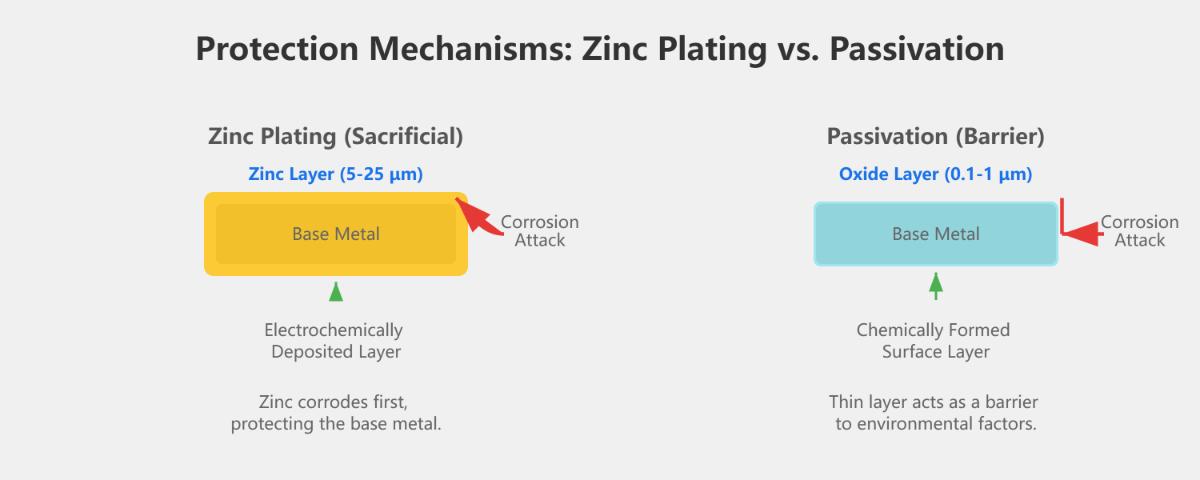
The primary difference between zinc plating and passivation lies in their processes and the type of protection they offer. Zinc plating involves electrochemical deposition of a zinc layer onto metal substrates, creating a sacrificial anode that provides robust corrosion resistance. This zinc layer, typically 5-25 μm thick, is particularly effective in harsh environments like marine settings.
In contrast, passivation is a chemical treatment applied to metals, often after zinc plating, to form a thin oxide or conversion coating (0.1-1 μm thick). This passive layer enhances the metal’s corrosion resistance by acting as a barrier to environmental factors. Passivation does not involve adding a significant material layer but rather modifies the surface chemistry to improve durability.
Which provides better corrosion resistance: zinc plating or passivation?
In terms of corrosion resistance, passivation typically provides superior protection compared to zinc plating alone. Zinc plating offers fundamental protection through barrier and sacrificial mechanisms, where the zinc layer acts as a physical shield and corrodes preferentially to protect the underlying metal. However, passivation enhances this protection by chemically treating the zinc-plated surface to form a denser, less reactive layer that resists oxidation and environmental contaminants. This conversion layer slows the corrosion of zinc itself, indirectly extending the protection of the base metal, particularly in harsh environments such as those with high humidity or salt exposure. Therefore, while zinc plating offers essential corrosion resistance, passivation improves durability and is recommended for applications requiring enhanced longevity and resilience against extreme conditions.
Can passivation be used without zinc plating?
Passivation cannot be effectively used without zinc plating when considering zinc-specific processes. The passivation treatment is designed to enhance the corrosion resistance of zinc-coated surfaces by forming a protective oxide or chromate layer. This layer stabilizes the zinc, preventing oxidation and extending the longevity of the zinc coating. While passivation can be applied to other metals like stainless steel and aluminum, these applications differ from the zinc-specific context. In summary, passivation is typically a supplementary process that requires a pre-existing zinc layer, such as from zinc plating or galvanizing, to function effectively. Without this layer, passivation alone is insufficient for standalone corrosion protection.
What are the environmental considerations for chromate passivation?
Environmental considerations for chromate passivation are significant due to the toxicity of hexavalent chromium (Cr⁶⁺), which is commonly used in traditional chromate passivation processes. Hexavalent chromium is a known carcinogen and poses severe health risks, including lung cancer and organ damage. It also presents considerable ecological harm, as evidenced by contamination incidents like the Hinkley, California case and the 2022 Tribar Technologies spill in Michigan.
Regulatory compliance is stringent, with global restrictions on Cr⁶⁺ due to its persistence in water systems and potential for bioaccumulation. Non-compliance can result in severe environmental contamination and significant legal and financial repercussions. Additionally, managing the hazardous wastewater generated by chromate processes requires specialized treatment, which increases operational costs and liability.
As an alternative, trivalent chromate passivation (Cr³⁺) is less toxic and aligns better with modern environmental standards, offering a safer option while maintaining adequate corrosion resistance. Other non-chrome coatings, such as cerium-based or zirconium-based passivates, are emerging but may present performance trade-offs.
How does regulatory compliance impact process selection?
Regulatory compliance significantly impacts the selection between zinc plating and passivation processes due to differing environmental and safety considerations. Regulations such as RoHS, REACH, and OSHA guidelines influence material and process choices by dictating permissible chemical usage and waste management. Zinc plating often involves hazardous substances like hexavalent chromium, requiring stringent safety measures and effluent treatment systems to meet compliance standards, thus increasing operational costs. In contrast, passivation typically uses less hazardous chemicals, simplifying compliance with environmental regulations and reducing waste management burdens.
Passivation is favored for its lower regulatory burden and is suitable for stainless steel and certain alloys, aligning well with industries prioritizing lean compliance strategies. However, its applicability is limited compared to zinc plating, which offers broader material compatibility and is essential in industries requiring high corrosion resistance, such as automotive and aerospace. Ultimately, process selection balances compliance requirements with specific industry needs, where zinc plating’s durability is indispensable, and passivation offers cost-effective compliance for select applications.
